Credits: Canva
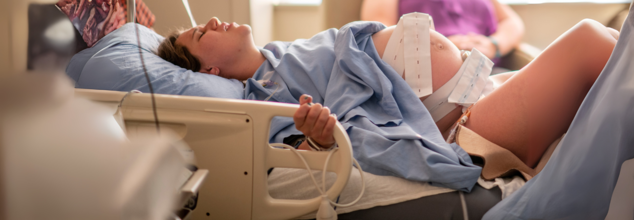
Peripartum Cardiomyopathy: What Kills Women During Childbirth?
Childbirth is usually considered a moment of happiness and beginnings, but for numerous women worldwide, it could become a life-or-death situation. Though excessive bleeding, infection, and hypertensive complications are well-known reasons for maternal mortality, an underemphasized yet life-threatening condition—peripartum cardiomyopathy (PPCM)—is slowly emerging as a silent killer. This unusual but dangerous manifestation of heart failure occurs in women late in pregnancy or shortly after delivery, frequently with symptoms that are indistinguishable from usual pregnancy discomforts.
According to the most recent UN global estimates, 303,000 women annually lose their lives as a result of complications in pregnancy or childbirth—an average of 830 women per day, or approximately one every two minutes. Most of these deaths could be averted with proper and timely medical attention. But extremes of inequality exist: two-thirds of maternal deaths happen in sub-Saharan Africa, with India and Nigeria alone contributing one-third of the world total.
Although the number of deaths worldwide has decreased by 44% since 1990, from 532,000 to 303,000, the achievement has not been commensurate with the size of global commitments over decades to cut down these figures. Most of the deaths remain underreported, especially in areas with weak healthcare systems and inadequate data collection mechanisms.
What Is Peripartum Cardiomyopathy?
Peripartum cardiomyopathy is a form of heart failure that occurs in the final month of pregnancy or within five months after delivery. It compromises the function of the heart muscle, making it less efficient at circulating blood throughout the body. Clinically, it appears as heart failure with low ejection fraction (EF < 45%) without any other established cause. Although the specific etiology of PPCM is not known, risk factors are advanced maternal age, Afro-American heritage, pre-eclampsia, chronic hypertension, and multiparity.
Although PPCM is uncommon—affecting between 1 in 1,000 and 1 in 4,000 pregnancies in the United States—it's a significant health risk when it does occur. It has been found to be more common in the southern parts of the U.S., but worldwide estimates are uncertain due to under-reporting and under-diagnosis.
One of the most perilous features of PPCM is how readily it can be confused with normal pregnancy symptoms. Fatigue, shortness of breath, swelling in the feet and ankles, and mild chest pain are all normal pregnancy occurrences. But for women with PPCM, these symptoms can indicate a heart in distress. Severer forms cause difficulty in lying down due to breathing (orthopnea), night-time breathing distress (paroxysmal nocturnal dyspnea), palpitations of the heart, and even decreased blood pressure or cardiogenic shock.
"The PPCM clinical syndrome is easily likely to be mistaken for late-gestational complains," states Consultant – Gynecology Dr. Madhu Bindhu. "It usually brings delays in diagnosis, which, being critical at times, causes anxiety. Earlier recognition, notably via echocardiographic screening, is responsible for better outcome improvement."
How Is PPCM Diagnosed and Treated?
Diagnosis often starts with a 12-lead ECG and a chest X-ray, but the definitive test is a 2D echocardiogram, which can verify left ventricular dysfunction. In complicated cases, a cardiac MRI may be performed to exclude other structural or inflammatory causes of heart failure.
Treatment is with a typical heart failure regimen, adjusted for pregnancy or postpartum status. This can be diuretics to decrease fluid accumulation, beta-blockers to manage heart rate, and ACE inhibitors, utilized postpartum. Anticoagulants can be given to avoid blood clots, a frequent complication in PPCM.
A treatment that is under investigation and shows promise is Bromocriptine, a prolactin inhibitor with disease-modifying effects. In severely ill patients, more advanced treatments like ECMO (extracorporeal membrane oxygenation), intra-aortic balloon pumps, or LVADs (left ventricular assist devices) are options.
Pregnancy, Delivery, and the Cardiomyopathy Equation
For pregnant women diagnosed with PPCM, delivery plans need to be tailored. Vaginal delivery is favored for hemodynamically stable patients, states Dr. Bindhu. But those with critical cardiac decompensation are advised to have cesarean sections at any gestational age.
Significantly, a multidisciplinary approach by cardiologists, obstetricians, and intensive care specialists is vital. Pregnancy heart teams dedicated to caring for complex PPCM cases can make a life-changing difference and lower mortality risks.
Outcome in PPCM is variable. Many women recover if treated appropriately, but some might have chronic heart failure or potentially life-threatening complications. Poor prognostic indicators are an ejection fraction less than 30%, dilatation of the left ventricle, biventricular dysfunction, prolongation of the QT interval, and non-Caucasian ethnicity.
Public education, early diagnosis, and enhanced access to prenatal care are the pillars of preventing maternal mortality due to PPCM. In resource-poor countries, enhancing diagnostic capacity and educating clinicians to diagnose PPCM is crucial.
Peripartum cardiomyopathy is still one of the least talked-about but most perilous maternal medical conditions in the world. As we continue to aim to decrease maternal death rates, particularly in low-resource environments, it is important that PPCM be part of the discussion. Every pregnant woman should have the means and attention that can identify and treat the condition in time—so motherhood can start in hope, not heartbreak.
Dr Madhu Bindhu is a Consultant – Gynecology at Manipal Hospital, Vijayawada in India

Credits: Canva
You Inherit Your Mother's Cortisol
New research suggests that babies born to mothers who experienced trauma during pregnancy—specifically the 9/11 attacks—may inherit altered stress responses due to changes in a key hormone: cortisol. Cortisol is commonly known as the body’s “stress hormone” and plays a major role in regulating blood pressure, blood sugar, and immune responses. But under extreme stress, the body’s cortisol system can become dysregulated—and this dysregulation may start before birth.
What is cortisol and why does it matter?
Cortisol is produced by the adrenal glands in response to stress. It helps the body manage challenging situations by raising blood glucose levels, increasing blood pressure, and aiding in immune function. Normally, cortisol levels rise when we’re under stress and return to normal afterward.
However, in situations of severe or prolonged stress, the body may start using up cortisol faster than it can produce it. This can lead to abnormally low cortisol levels—a condition linked with fatigue, anxiety, and even immune system disorders.
The 9/11 study: A natural experiment in trauma
A unique study led by researchers from the University of Edinburgh and Mount Sinai School of Medicine focused on 38 women who witnessed the September 11, 2001 attacks on the World Trade Center. One year after the event, these women and their babies were tested for cortisol levels.
The findings were surprising. Women who developed post-traumatic stress disorder (PTSD) following the attacks had significantly lower levels of cortisol—so did their babies. This suggests that the trauma experienced by the mother was, in some way, biologically passed on to the child.
Timing matters: Third trimester appears most sensitive
Interestingly, the effect was strongest in women who were in their third trimester of pregnancy during the attacks. According to Jonathan Seckl of the University of Edinburgh, this detail points to a biological explanation rather than simply differences in parenting style. “That seems much more biological than about delivering care,” he said.
This timing is crucial because during the third trimester, a baby’s brain and hormonal systems are undergoing rapid development. Any disruptions during this period—such as a sudden drop in cortisol levels—could “program” the baby’s stress response system for life.
Nature, nurture, or both?
While earlier studies linked maternal trauma to children’s stress disorders, many assumed this was due to storytelling or emotional modeling—children learning fear and stress from their parents’ accounts of traumatic events. But the findings from this study challenge that idea.
Since the babies were only around one year old during testing, they were likely too young to have absorbed their mothers’ traumatic stories or behaviors in depth. Instead, the low cortisol levels may reflect biological programming in the womb or even shared genetic vulnerabilities to stress.
Health risks associated with low cortisol
Experts like Andrew Steptoe of University College London caution that while the connection between cortisol and stress is complex, low cortisol levels aren’t necessarily benign. “The most obvious [problem] is regulation of the immune system,” he noted. Chronic low cortisol can leave individuals more vulnerable to infections, inflammation, and mood disorders later in life.
While more research is needed to fully understand how maternal trauma affects fetal development, the 9/11 study adds to growing evidence that stress during pregnancy can have long-term biological effects on children. These findings emphasize the importance of providing psychological support to expectant mothers—especially in times of crisis—to help buffer both them and their babies from lasting harm.

Credits: Canva
Micronutrient Deficiencies Could Mimic Learning Disorders In Kids, Parents Here’s What You Can Undo
If your child is having trouble paying attention in class, remembering instructions, or showing signs of poor academic performance, it's natural to wonder about a learning disorder. But suppose the real culprit is hiding in their lunchbox?
A shocking body of research now suggests that micronutrient deficiencies among children can hauntingly mimic the symptoms of cognitive delay, behavioral issues, and even learning disabilities. What may seem to be a developmental or neurological disorder may well be traced back to a far more preventable source: a lack of vital vitamins and minerals.
Hunger is not just about empty stomachs—it's about the lack of quality nutrients the body needs to function and grow. In the United States alone, more than 13 million children—about 18%—live in food-insecure households. That means almost one in five children who don't have regular access to nutritious meals.
In accordance with a number of studies, children who develop in food-insecure homes are likely to enter school behind. They are less likely to be developmentally prepared for kindergarten, and are likely to suffer from undiagnosed iron deficiency anemia—an problem research has determined to weaken memory and social functioning even a decade later.
Their performance at school declines with lower test scores on standardized tests, slower reading development, and more emotional and behavioral problems. The majority of these children receive inappropriate diagnoses of learning disabilities when, in fact, they are struggling due to nutrient deficiencies.
It's easy to get confused between the symptoms of micronutrient deficiency and learning or attention disorders. Children can appear sluggish, daydreamy, irritable, or lack interest in learning—symptoms most typically associated with ADHD, anxiety, or developmental delay.
A learning disorder is where the brain perceives information in a different manner, which holds a child back from learning or applying certain abilities effectively—yet they might be of average or above-average intelligence. This neurologic condition creates a noticeable deficit between the potential and existing academic performance of a child. Learning-disabled children may experience trouble with reading, writing, math, or attention, but the point is that these troubles are not linked with intelligence or effort. Early recognition and intervention are the most effective means of allowing children to acquire the skills required to be successful in school as well as everyday life.
But as Nutritionist Aman Puri says, it begins in the kitchen, "Good nutrition is the foundation for a child to grow and develop to his/her full potential. While motor and cognitive function begin unfolding during early childhood, deprivation of essential nutrients delays or impedes these natural milestones."
In particular, iron, zinc, iodine, and vitamin B12 deficiencies have actually been specifically implicated in cognitive dysfunctions, trouble remembering things, and low IQ levels. Even in certain cases, these deficiencies aren't visibly apparent until a great deal further down the line—when the academic or behavioral issues suddenly begin surfacing.
How Each Nutrient Affects Your Child's Brain?
Iron is needed to deliver oxygen to the brain. Deficiencies reduce red blood cell production and hemoglobin, impacting cognitive development directly.
Zinc is linked with attention span, problem-solving, and memory consolidation. Deficiencies can slow learning significantly.
Iodine is needed for early brain development. Iodine-deficient children have lower intelligence test scores and are at risk for goitre.
Vitamin B12 supports neurological function. Deficiency can retard motor skills and mimic ADHD and language processing disorder symptoms.
"Deficiencies may or may not initially present with any symptoms at all," says Puri. "But over time, they may influence brain growth so much so that it becomes easy to equate them with clinical learning disability."
The Problem With Modern Diet is More Than Junk Food
Even in households where food is abundant, nutrition can be lacking. Between processed foods, fast food, and sugary drinks, children are taking in calories—but not necessarily the essential nutrients they require. Throw in hectic parental schedules and a lack of nutritional knowledge, and the outcome is a recipe for malnourishment, even in middle-income households.
Malnutrition is the cause of nearly half of the deaths of all children under age five, and the World Health Organization (WHO) tells us so. And to those few who survive it, developmental lags and learning issues become the normal challenge to deal with.
Simple Steps for Smarter Nutrition Parents Can Follow
So what can be done? First, parents can take back control by providing a well-balanced, micronutrient-dense diet. Puri recommends introducing fortified foods and natural sources of essential nutrients early on—namely, the first five years of life when the brain grows most rapidly.
Nutrients to include:
Iron: Leafy greens, legumes, eggs, lean meats
Zinc: In milk products, seeds, and whole grains
Iodine: From iodized salt, seaweed, and milk
Vitamin B12: Found in fish, eggs, milk, and fortified cereals
Folate, calcium, vitamins A, D, and E: Best found on a plate of rainbow hues
"Nutrition education strategies must begin with maternal and child health programs," says Puri. "Mothers must be educated on the long-term consequences of micronutrient deficiencies and the benefits of early dietary intervention."
Before rushing to label your child with a learning disorder, take a closer look at their plate. The brain, like any other organ, responds well to optimal fuel. And occasionally, the solution to deficient focus, mood swings, or learning lags is as elementary—and as life-changing—as an upgrade in nutritional quality.
Aman Puri is a Nutritionist and Founder, Steadfast Nutrition with an expertise as a Sleep Science Coach and in Sports Psychology.

Credits: Canva
1 In 3 Maternal Deaths Happen 6-Weeks Post Delivery, Finds Study
A new study in JAMA Network Open, titled Pregnancy-Related Deaths in the US, 2018-2022 revealed one of the most overlooked fact, which is that nearly one-third of maternal deaths in the US occurred more than six weeks after childbirth. Why is this a big concern? The reason for it being alarming is because six weeks post giving birth is generally considered a "safe zone" for new mothers. The study therefore highlights the critical need for continued maternal care, which is important not just during pregnancy, but for an entire year after delivery.
A New Understanding For Maternal Health
The study is among the first that tracked maternal complications from the stage of conception to one year after birth. The study put in use the data collated by the Centers for Disease Control and Prevention (CDC), and found that pregnancy related deaths in the US rose nearly 28% from 2018 to 2022. The numbers also peaked in 2021, when COVID-19 was at its peak, however, the numbers slightly declined the following year.
This has brought in a new shift in understanding the challenges with the traditional six-week postpartum care model. It also throws light on the need for an extended support. “Our study illustrates why we can’t take our eyes off maternal health,” said Dr. Rose Molina of Harvard Medical School. “Women need access to high-quality care from the moment of conception to a full year after birth.”
What Are Late Maternal Deaths?
Late maternal deaths are defined as those that occur six week to one year after childbirth. These deaths also take place after the standard six-week checkup, which is often considered as the final appointment for women after their delivery.
The American College of Obstetricians and Gynecologists (ACOG) recommends that new mothers should see their doctor within the first three weeks after their childbirth. This should also be followed by personalized care and a comprehensive checkup by 12 weeks. High-risk patients, such as those with hypertensive disorders, may require even earlier visits.
Experts also emphasized that this shift is important as crossing the six-week mark is no longer the safe zone as it was thought before. Experts are now asking patients to book appointments even without complications.
What Are The Leading Causes Of Late Maternal Births?
The study found that cardiovascular disease was the leading cause of maternal deaths over all. This was especially the case in late postpartum period. Other contributors were cancer, mental and behavioral disorders, and drug or alcohol related deaths. Accidents and homicides were not counted in the study.
The findings highlighted that pregnancy can in fact strain heart and also aggravate pre-existing conditions like high blood pressure. These are issues which are seen more in younger adults. Experts also explain that these issues disproportionately affect women aged 25 to 39.
Deaths And Disparities
The study also documented disparities among the ethnic and racial communities. Native American and Alaska Native women died at the rate of 3.8 times higher than White woman. Whereas, Black women had rates 2.8 times higher. The lowest death rates were recorded among Hispanic and Asian women.
State-by-state, death rates varied dramatically. Alabama had the highest maternal mortality rate, followed by Mississippi. California and Minnesota had the lowest.
© 2024 Bennett, Coleman & Company Limited

