- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
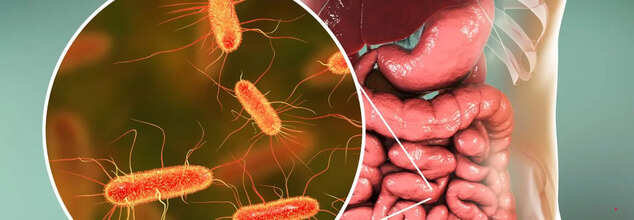
How Does Your Gut Microbiome Impact Your Overall Health?
Credit: Canva
Your body hosts trillions of bacteria, viruses, and fungi, collectively known as the microbiome. While some bacteria are linked to disease, many play essential roles in supporting your immune system, heart health, weight management, and overall well-being. This article delves into the significance of the gut microbiome and its impact on health.
What Is Gut Microbiome?
Microorganisms, or microbes, reside in various parts of your body, but the majority are concentrated in the intestines, particularly in the cecum, a part of the large intestine. This collection of microbes is referred to as the gut microbiome.Interestingly, bacterial cells outnumber human cells in the body, with approximately 40 trillion bacterial cells compared to 30 trillion human cells. With up to 1,000 species of bacteria present in the gut, each plays a distinct role. While most bacteria contribute positively to health, some can be harmful. Together, these microbes weigh around 1–2 kilograms, functioning almost like an additional organ essential for overall well-being.
How Does Gut Microbiome Influence Your Health?
The relationship between humans and microbes has evolved over millions of years, with the gut microbiome playing a crucial role from birth. Initial exposure to microbes occurs during birth, and some evidence suggests that exposure begins in the womb. As the microbiome diversifies, it starts influencing key bodily functions:
Digestion of breast milk: Beneficial bacteria like Bifidobacteria help break down essential sugars in breast milk, supporting infant growth.
Fiber digestion: Some bacteria process fiber into short-chain fatty acids, which contribute to gut health and reduce risks of obesity, diabetes, and heart disease.
Immune system regulation: The gut microbiome interacts with immune cells, influencing how the body responds to infections.
Brain health: Emerging research suggests a link between the gut microbiome and brain function, potentially affecting mental health and neurological processes.
Gut Microbiome And Weight Management
An imbalance between beneficial and harmful microbes, known as gut dysbiosis, may contribute to weight gain. Studies on identical twins—one with obesity and the other without—suggest that microbiome composition plays a role in body weight independent of genetics. Additionally, animal studies indicate that gut bacteria can influence weight gain, even when calorie intake remains constant.
Probiotics, beneficial bacteria found in supplements and certain foods, can help restore gut balance and support weight loss, though their effects may be modest.
Gut Health And Disease Prevention
The gut microbiome plays a vital role in preventing and managing conditions like irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). Imbalances in gut bacteria may lead to bloating, cramps, and digestive issues. On the other hand, beneficial bacteria like Bifidobacteria and Lactobacilli help strengthen the intestinal lining, reducing the risk of gut-related disorders.
Impact On Heart Health
Research suggests that the gut microbiome influences heart health by affecting cholesterol levels and blood pressure. Certain harmful bacteria produce trimethylamine N-oxide (TMAO), a compound linked to blocked arteries and heart disease. However, probiotics, particularly those containing Lactobacilli, may help reduce cholesterol levels and promote cardiovascular health.
Blood Sugar Regulation And Diabetes Risk
The gut microbiome also plays a role in regulating blood sugar levels. Research on infants genetically predisposed to type 1 diabetes indicates that gut microbiome diversity declines before disease onset. Furthermore, individual variations in gut bacteria may explain why people experience different blood sugar responses to the same foods.
Connection Between Gut And Brain
The gut is physically connected to the brain through nerves, and certain bacteria help produce neurotransmitters like serotonin, which influence mood and mental health. Studies indicate that people with mental health disorders often have different gut bacteria compared to those without such conditions. Additionally, some probiotics have shown promise in alleviating symptoms of depression and anxiety.
Tips For Healthier Gut Microbiome
Maintaining a balanced gut microbiome is crucial for overall health. Here are some strategies to support gut health:
- Eat a diverse range of foods: A varied diet rich in fiber, legumes, and fruits promotes microbial diversity.
- Consume fermented foods: Yogurt, kefir, and sauerkraut contain probiotics that enhance gut health.
- Limit artificial sweeteners: Some artificial sweeteners can encourage the growth of harmful bacteria.
- Include prebiotic foods: Bananas, oats, and asparagus contain fibers that nourish beneficial bacteria.
- Breastfeed infants when possible: Breastfeeding promotes a healthier gut microbiome in babies.
- Choose whole grains: Whole grains contain fiber and beneficial compounds that support digestive health.
- Opt for a plant-based diet: Vegetarian diets may reduce inflammation and support a healthier gut microbiome.
- Consume polyphenol-rich foods: Green tea, dark chocolate, and olive oil contain compounds that foster beneficial bacteria.
- Take probiotics if needed: Probiotic supplements can help restore gut balance after disruptions, such as antibiotic use.
- Use antibiotics cautiously: While antibiotics can be life-saving, overuse can harm beneficial gut bacteria.
UK Sees 11% Drop In Cancer Death Rates Over The Past Decade

Cancer deaths in the United Kingdom have dropped to their lowest recorded levels, according to new data from the charity Cancer Research UK. The figures show that cancer death rates have fallen by 11 per cent in the past decade, reflecting progress in early detection, screening, treatment and prevention.
Researchers estimate that around 247 people in every 100,000 in the UK now die from cancer each year. This is a significant decline from the peak recorded in 1989, when about 355 people per 100,000 died annually from the disease. Overall, that represents a 29 per cent reduction over the past few decades.
Experts say this steady improvement is the result of sustained scientific progress, improved healthcare systems and public health measures that target risk factors such as smoking.
Major Declines In Several Common Cancers
The new data highlights falling death rates across several major cancers. Ovarian cancer deaths have dropped by 19 per cent over the past ten years, up to 2024. Lung cancer deaths have fallen by 22 per cent during the same period, reflecting the long-term impact of reduced smoking rates and better treatment options.
Deaths from stomach cancer have seen one of the most dramatic improvements, dropping by 34 per cent in the past decade. Bowel cancer deaths have decreased by six per cent, while breast cancer deaths have fallen by 14 per cent.
Other cancers have also seen notable declines. Cervical and prostate cancer deaths have both dropped by 11 per cent. Deaths from leukemia are down by nine per cent, while esophageal cancer deaths have fallen by 12 per cent.
Experts say improved diagnosis, new therapies and better awareness are playing a key role in these trends.
Screening And Vaccination Making A Difference
One of the biggest public health successes has been the decline in cervical cancer deaths. Since the 1970s, deaths from cervical cancer have fallen by around 75 per cent in the UK. Health experts attribute much of this progress to the national cervical screening programme run by the NHS.
Screening helps detect abnormal cells early, allowing treatment before cancer develops or spreads.
Another major contributor is the human papillomavirus vaccine, commonly known as the HPV vaccine. The vaccine protects against the virus responsible for most cervical cancer cases. It is routinely offered to schoolchildren in the UK, and since its introduction in 2008, at least 6.5 million young people have received it.
Public health experts believe the vaccine will continue to reduce cervical cancer rates in the coming decades.
Some Cancer Death Rates Still Rising
Despite the overall progress, the data also shows worrying increases in deaths from certain cancers. Gallbladder cancer deaths have risen by 29 per cent, while deaths from eye cancer have increased by 26 per cent.
Liver cancer deaths are up by 14 per cent, and kidney cancer deaths have risen by five per cent. Meanwhile, death rates for thyroid cancer, pancreatic cancer and melanoma have remained largely unchanged.
Another important trend is that the total number of people dying from cancer continues to rise. This is largely due to population growth and an ageing population, as cancer risk increases with age.
Calls For More Research And Clinical Trials
Experts say the long-term decline in cancer deaths reflects decades of medical research and innovation. However, they stress that continued investment is necessary to sustain progress.
Cancer Research UK researcher Dr Sam Godfrey said the figures show the impact of scientific breakthroughs over many years. He has called on the government to support more clinical trials and ensure that NHS staff have enough time and resources to take part in life saving research.
Public health policies such as smoking bans, along with screening programmes and vaccines, are also credited with helping drive down cancer deaths across the country.
Scrolling Your Phone While In Toilet Can Spike Your Risk Of Hemorrhoids By 46%: Study

Credit: Canva
Are you in the habit of catching up on social media or news updates while sitting on the pot? A new study shows you may be "unintentionally" spending extra time and increasing your risk of developing painful hemorrhoids by 46 percent.
The study, published in the open-access journal PLOS One, explained that getting distracted by news or social media can increase pressure on sensitive anal tissues, which leads to hemorrhoids.
Hemorrhoids, also called piles, are swollen and inflamed veins around your anus or in your lower rectum.
"Using a smartphone while on the toilet was linked to a 46 percent increased chance of having hemorrhoids. We're still uncovering the many ways smartphones and our modern way of life impact our health,” Trisha Pasricha, from the Beth Israel Deaconess Medical Center in the US.
“It's possible that how and where we use them -- such as while in the bathroom -- can have unintended consequences," she added.
For the study, the researchers examined data from colonoscopies of 125 adults in America, and conducted an online survey to understand their lifestyle habits and behavior while using the toilet.
Two-thirds of the participants reported using their smartphones while on
the toilet. Compared with those who did not, endoscopists found that they had a 46 percent higher risk of hemorrhoids.
Longer Toilet Time And Mobile Scrolling
More than a third of bathroom smartphone users reported spending more than five minutes there during a single visit -- reading news (54.3 percent), or browsing social media (44.4 percent).
In comparison, just 7.1 percent of non-users reported staying that long.
"Smartphone use may unintentionally extend the time people spend sitting on the toilet. Sitting for longer periods could increase pressure on tissues in the anal region, which may contribute to the development of hemorrhoids,” the researchers said.
Pasricha suggested individuals leave smartphones outside the bathroom to understand the actual time it takes for a bowel movement.
"If it's taking longer, ask yourself why. Was it because having a bowel movement was really so difficult, or was it because my focus was elsewhere?" she said, calling for more studies.
Hemorrhoids: The Symptoms, Risk Factors
Studies estimate that hemorrhoidal disease affects 40% of people all over the world, and it is one of the most common diseases in the anorectal region.
The two types of hemorrhoids are:
- external hemorrhoids -- under the skin around the anus
- internal hemorrhoids -- in the lining of the anus and lower rectum
- bright red rectal bleeding during bowel movements,
- anal itching or irritation, pain or discomfort (especially while sitting),
- swelling or hard, tender lumps around the anus
- straining during bowel movements
- sitting on the toilet for too long
- chronic constipation or diarrhea
- eating low fiber foods
- older age >50
- pregnancy
- lifting heavy objects
- consume 25-35g of fiber daily,
- drink 6-8 glasses of water,
- avoid straining,
- limit toilet time to under five minutes
- do not delay bowel movements,
- maintain hygiene.
Black Rain Over Iran's Capital Tehran Sparks Health Fears

Credits: X/Twitter
Iran's capital Tehran was engulfed in a black cloud of toxic smoke. This also unleashed a black rainfall on Sunday after overnight Israeli strikes on several fuel depots caused fires to burn for hours. Images have come from across the city of Tehran. These images show thick black smoke from the fires hanging over it. Residents have also reported difficulty breathing and oil-tainted rainfall staining everything around them.
As per a TIME report, Iran's Red Crescent Society warned the residents of Tehran and the surrounding region that the rainfall after the strikes could be "highly dangerous and acidic", and could cause "chemical burns of the skin and serious damage to the lungs".
Many have complained about breathing problems, along with headache, feeling dry and sore lips, and feeling like burn in the eyes and constant itch in the throat.
What Does The Toxic Rain Fall Contain?
Iran's Red Crescent Society issued statements on Telegram that the rain could be contaminated with "toxic hydrocarbon compounds" as well as "sulfur and nitrogen oxides".
What Are The Health Risks?
As per a report by The Conversation, people exposed to the black smoke in Iran could experience headaches or difficulty in breathing, especially if they have asthma or a lung disease.
People who are more prone to health issues are older people, young children, anyone with disabilities and pregnant women. This could also lead to lower birth weights.
Since the thick black cloud from all the burning could increase the PM2.5 or the ultrafine particles, known as particulate matter, it could also increase cancer risks, along with neurological conditions and cardiovascular conditions.

The toxic rain could further pollute the natural waterways and drinking water sources. A photo shared by Iran's Red Crescent shows a healthcare worker's uniform covered in black droplets from the rain.
The "rain drops" are tainted with oily residue and could lead to skin problems, and if inhaled, it could also lead to serious medical crisis, noted Jim NR Dale, a senior meteorologist at British Weather Services.
It may also carry carcinogenic polycyclic aromatic hydrocarbons (PAHs) along with heavy metals that are released when construction materials burn and then remain suspended in the air.
As acidity increases, natural water bodies such as rivers and lakes can become too hostile to support life. When the pH of water drops below 5, most fish cannot survive, and at pH 4, a lake is often described as a “dead water body” because almost no living organisms remain.
Acid rain also harms the soil. It reduces calcium levels, an important nutrient for plants, and makes it easier for toxic aluminium to leach into water sources, further threatening ecosystems.
© 2024 Bennett, Coleman & Company Limited

