- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
FDA Approves ‘Spravato’ Nasal Spray As A Standalone Depression Treatment

(Credit-Canva)
Educating people about the importance of mental health has been one of the best steps taken. Not so long ago, people did not believe in depression, let alone treat people who have depression correctly. But in recent years we have seen a rise in people who are taking mental health more seriously and working towards making their lives and the people around them better. We also have reached a point where we have medication and treatment options for not just people with depression, but anxiety, BPD, schizophrenia etc. And now FDA has broadened the variety of medicines that are available to people to fight depression! The U.S. Food and Drug Administration (FDA) has broadened its approval for Johnson & Johnson's nasal spray, Spravato esketamine, allowing its use as a monotherapy for severe depression, as announced by the company on Tuesday. This decision marks a significant development in the treatment landscape for individuals with major depressive disorder (MDD).
Spravato was first approved in 2019 to be used with regular antidepressants for people whose depression wasn't getting better with other medicines. It was like an extra treatment that acted almost like a strengthener. Later, it was also approved for people who were thinking about suicide or hurting themselves, because it can work quickly in those urgent situations. Now, doctors can give Spravato by itself, without the person having to take a regular antidepressant pill too. This is a big change and gives doctors and patients more choices.
What This Means For Patients
Experts suggest that with this approval they can now make more personalized medicine for their patients, as we know, everyone takes medicine differently and there is no one-way street for treatment. Doctors now have more ways to treat depression. Some people might do better with just Spravato, while others might still need other medicines too. This personalized way of treating people is really important because everyone's different. This change lets doctors choose the best treatment based on what each person needs, which could help people get better faster.
What Measures Have Been Taken For Patient Safety?
The FDA approved Spravato for use on its own because studies showed it works well in this way. People who used Spravato got better faster than those who didn't in clinical trials. The medicine works on a chemical in the brain called glutamate, which is involved in mood regulation. Spravato is related to ketamine, which is sometimes used illegally as a recreational drug. While both affect glutamate, they are not identical. The clinical trials provided the necessary evidence for the FDA to expand the drug's approved use.
Because Spravato has the potential for misuse and some side effects, it's only available through a special program called a REMS which is the Risk Evaluation and Mitigation Strategy program. The process here is that people who will be given the dose of medicine will be kept under close supervision. The doctors will check on them and their vitals every two hours and see how well the patients were responding to the medicine. This is to make sure it's used safely and to monitor for any adverse reactions, sometimes people don’t respond well to medication, it can also be dangerous if it reacts differently to others. The REMS program is a key part of ensuring patient safety and responsible use of this medication. This controlled distribution system helps to minimize the risk of misuse and ensures proper medical supervision.
Diseases Are Spreading Fast- But Why Isn’t The CDC Saying Anything?
In an age where outbreaks can cross borders in hours and pandemics reshape entire economies, timely and transparent communication is not just helpful—it’s critical. The U.S. Centers for Disease Control and Prevention (CDC), long considered the global gold standard for public health messaging, now finds itself disturbingly muted.
Since the early days of the Trump administration, vital communication channels that once buzzed with disease updates, outbreak alerts, and public health advisories have gone noticeably dark. The silence is not just an administrative hiccup—it’s a systemic failure that public health experts fear could have devastating consequences for Americans and beyond.
For decades, the CDC operated with a clear mandate—to deliver science-based information to clinicians, researchers, policymakers, and the public to contain the spread of disease and save lives. From newsletters on diabetes and arthritis to emergency health alerts about disease outbreaks, the agency was a well-oiled communications machine.
That changed abruptly in January of Trump’s first term. According to internal sources and an NPR investigation, most of the CDC’s newsletters ceased distribution. The Health Alert Network (HAN)—which had served as a critical pipeline between the CDC and healthcare providers—has not issued a single alert since March. Content once overseen by CDC communicators is now subject to approval by the Department of Health and Human Services (HHS), which has taken over ownership of the CDC’s social media platforms.
“We are functionally unable to operate communications,” a current CDC employee admitted. “We feel like our hands are tied behind our backs.”
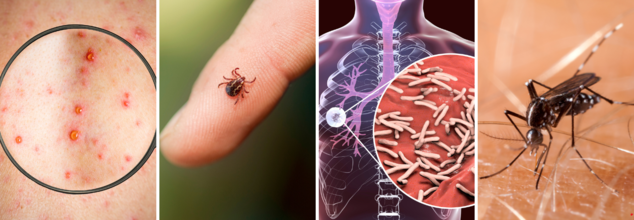
This communications bottleneck couldn’t have come at a worse time. The world is seeing an uptick in both new and re-emerging infectious diseases—from resurgent measles outbreaks in Europe and the U.S. to mosquito-borne threats like dengue and chikungunya in tropical zones. In Africa, Ebola and mpox cases are climbing. In Asia and the Americas, diseases like Zika and Japanese Encephalitis continue to circulate.
Meanwhile, here in the U.S., hepatitis outbreaks, chronic illness complications, and listeria contamination events continue with little to no public guidance from the CDC. A lack of clear information impedes early detection and prevention—two of the most crucial elements of effective public health response.
As Kevin Griffis, former CDC communications director, warned: “Public health functions best when its experts are allowed to communicate the work that they do in real time, and that’s not happening. That could put people’s lives at risk.”
Major Diseases That Are Spreading Across The World
While the CDC’s public channels remain conspicuously quiet, dozens of diseases are gaining ground, many of them preventable or containable with the right knowledge:
Dengue and chikungunya, spread by infected mosquitoes, are on the rise in Central and South America.
Measles, once nearly eradicated in the U.S., is resurging due to declining vaccination rates.
Tuberculosis (TB), polio, and Hepatitis A, B, and C remain significant threats in urban and rural populations.
Norovirus and listeria, both foodborne, continue to cause nationwide outbreaks.
Respiratory illnesses such as RSV and influenza still threaten vulnerable populations, especially children and the elderly.
Without timely updates and education from authoritative sources like the CDC, people are less equipped to take action—whether that means vaccinating their children, recognizing early symptoms, or preventing transmission.
Why Is CDC Not Sharing Health Updates?
Interviews with current and former CDC employees reveal a culture of fear and stifled autonomy. Workers are no longer allowed to post health facts or emergency updates without prior approval from HHS. Many posts are either delayed or blocked entirely—often stripped of critical details. And layoffs have further weakened the agency’s digital team.
Between February and April, internal newsletters were halted, social media activity plummeted, and even the CDC’s hallmark Morbidity and Mortality Weekly Report missed crucial publication dates.
In one shocking example, the CDC’s main Facebook page, which used to post multiple times daily, went silent for over a month. Even basic health updates—like how to care for a newborn or when to screen for colorectal cancer—have vanished from public view.
For a country that leads the world in biomedical innovation, the quieting of the CDC is a profound step backward. When transparency disappears from health communication, disinformation thrives. Public trust erodes. People delay seeking care. And diseases spread more easily, unchecked.
Moreover, the CDC’s influence goes beyond U.S. borders. International health agencies, global researchers, and low-resource countries depend on CDC data to calibrate their own responses. With the CDC effectively muzzled, the ripple effect could exacerbate global health inequities.
There is growing pressure from public health advocates, former CDC leaders, and international organizations for the U.S. government to restore autonomy to the CDC’s communications arm. This means not only reinstating internal decision-making powers but also rehiring expert teams and reactivating critical communication platforms.
Dormant For 50 Years, Woman Dies From Extremely Rare Brain Disease Decades After Childhood Hormone Shot
Credits: Canva
An extraordinary case reveals the shocking latency of prion disorders and poses international public health issues decades since infected hormone treatment ceased. A 58-year-old U.S. woman has succumbed to iatrogenic Creutzfeldt-Jakob disease (iCJD)—a lethal neurodegenerative and incurable brain disorder—almost 50 years after she was treated with cadaver-derived human growth hormone (chGH) during childhood. Her case, reported in Emerging Infectious Diseases by researchers at the University of Colorado, contributes to the increasing but uncommon cluster of iCJD cases associated with hormone treatments received years ago.
The woman was only seven years old in 1971 when she first received growth hormone therapy under the National Hormone and Pituitary Program (NHPP), which supplied cadaver-based hormones to address childhood growth failure. Her exposure lasted for more than nine years—a duration that would later be pivotal to understanding the long latency of the disease that would kill her.
The patient first came to attention with a two-week course of tremor and imbalance. Although her initial clinical workups revealed nothing calamitous—an unremarkable MRI scan and lackluster lab results—her case was taken downhill. In a matter of one month, she had progressively worsening tremor, urinary incontinence, dysarthria, and ataxia. Shortly thereafter, she became hyperekplexic, rigid, and comatose. A positive prion protein test sealed the diagnosis.
Her case is the 36th documented case of iCJD in U.S. recipients of chGH distributed by NHPP and the 254th overall. This case is unusual in that it has an exceptionally long latency period—some 48.3 years since first exposure.
What's Prion Disease?
Prion diseases, including CJD, are the result of abnormally folded proteins that initiate a cascade of misfolding in normal brain proteins. The chain reaction causes rapid neurodegeneration. Prion diseases are always fatal, and there is currently no cure. Prions are extremely resistant to standard methods of sterilization, making them especially treacherous in a healthcare environment.
What distinguishes iCJD from other types—sporadic or genetic—is where it came from: direct transmission through medical procedures. Here, transmission was through cadaver-derived hormone therapy, which is now outdated, but was commonplace during the middle part of the 20th century prior to the advent of recombinant biosynthetic hormones in 1985.
Latency that Defies Medical Precedent
One of the most chilling features of this case is the lengthy latency of the disease. The latency period, according to scientists, can vary anywhere from several years to over five decades. Determining factors for the duration include dose, route of administration, and recipient genetics—most notably a particular polymorphism of the PRNP gene (codon 129), which this patient had. This genetic mutation is linked with prolonged incubation of disease in acquired prion disease.
Four latency estimations were used in this case, but the most accurate—measuring from the midpoint of pre-1978 hormone treatment to symptom onset—yielded a 48.3-year latency. This lengthy dormancy makes iCJD uniquely difficult to track and almost impossible to prevent in retrospect.
As the U.S. NHPP was closed down shortly after confirmation of the iCJD association in the mid-1980s, this example highlights the long-term public health consequences of historical medical practices. Based on the study, about 7,700 children received chGH in the U.S., and over 250 have developed iCJD worldwide.
The investigators note that U.S. lots of chGH probably contained less prion contamination than foreign versions. Furthermore, purification procedures added in 1977 may have mitigated the risk but not removed it.
Curiously, experimental research with nonhuman primates identified that contamination of NHPP chGH lots was both infrequent and randomly distributed. Yet even these minimal amounts were sufficient to cause deadly infections decades later.
Diagnosing the Undiagnosable Disease
Diagnosis of prion diseases continues to be elusive and is usually only possible after death. In this instance, high-tech diagnostics—such as real-time quaking-induced conversion assay and cerebrospinal fluid tests—played a vital role in detecting the presence of prions. Increased levels of tau and 14-3-3 protein in cerebrospinal fluid also helped make the diagnosis. Autopsy diagnosis was made by Western blot and immunohistochemistry.
However, the window for treatment is practically nonexistent. Symptoms of prion diseases develop and advance rapidly, with the majority of patients dying in a matter of months from diagnosis.
With long latency periods and the extensive previous use of chGH, experts caution that there are still likely to be more cases of iCJD to come. This potential necessitates ongoing surveillance and retrospective patient follow-up.
This case is a stark reminder of the ability of medicine to heal and to harm. Although present-day biosynthetic hormone therapy is safe, the remnants of past methods still linger in modern healthcare. While science may continue to push forward, this tale emphasizes the need for careful monitoring, ethical treatment procedures, and extended patient follow-up—decades after the initial dose is administered.
Key Points About Prion Disease
- Prion conditions such as iCJD are very rare but always deadly.
- Diagnosis is difficult and confirmed only post-mortem in many cases.
- Latency can range from decades, making it difficult for public health intervention.
- There is no treatment, and healthcare is palliative at best.
In the meantime, the best protection is still awareness and prevention.
Humid, Heat, And Untimely Heavy Rains Could Lead To These Health Concerns

Credits: Canva
A lot is happening in Delhi and the regions around it. Delhi-NCR while is battling with extreme heat, heatwave, and humidity. In new events, Delhi-NCR is also witnessing untimely rains. While heatwave and humidity already have its own health issues, untimely rain further increases infection risks.
Humidity And Skin Diseases
Humidity—the moisture in the air—can be both friend and foe for your skin. On one hand, it boosts hydration and helps dry skin retain moisture. But on the other, it also increases oiliness, clogs pores, and creates the perfect breeding ground for breakouts, fungal infections, and flare-ups of eczema and psoriasis. If you notice persistent irritation, redness, or breakouts, it might be time to tweak your skincare routine or consult a dermatologist.
What Happens at the Skin Level
The skin’s outermost layer (stratum corneum) acts as a barrier to keep moisture in. In humid conditions, this layer absorbs water from the air, helping it stay hydrated. But there's a catch—your skin may start producing more oil (sebum), making it appear greasy and prone to acne, especially if you already have oily or combination skin. Humidity also disrupts the skin’s natural balance, leaving it more sensitive and reactive.
Heat and the Body’s Cooling Crisis
Hot and humid weather slows down the body’s cooling system. When sweat doesn’t evaporate easily, it increases internal heat, leading to heat exhaustion or even heatstroke. The World Health Organization warns that this can trigger heart, kidney, and mental health issues, especially in people with chronic illnesses. It also makes it harder to concentrate or work, putting strain on physical and mental productivity.
Rain and the Rise of Disease
Untimely rains, especially in urban spaces like Delhi, bring another set of problems. Waterlogging and stagnant puddles become breeding grounds for mosquitoes, pushing up cases of dengue, malaria, and chikungunya. Dengue, for instance, can lead to dangerously low platelet levels, while chikungunya is known for severe joint pain.
Monsoon's Hidden Health Hazards
The risk doesn’t stop at mosquito-borne diseases. Rains also increase the chance of waterborne infections like typhoid, hepatitis A and E, and leptospirosis (caused by exposure to floodwater contaminated with animal urine). Skin infections, particularly fungal ones, worsen in humid, unhygienic conditions—especially if feet remain damp for long. Cases of eczema flare-ups, candida infections, and even foot-related complications like gangrene rise during this season.
© 2024 Bennett, Coleman & Company Limited

