
Credits: Canva
Whooping Cough Cases Are Rising Across US- How Long Is The Infection Contagious?
Whooping cough cases are skyrocketing at a record rate across US, from Texas to Michigan, health departments are seeing a rise not seen in over a decade and it's not just the numbers that have experts alarmed. The most vulnerable are babies under the age of one, and in some tragic cases, the disease has been fatal.
State and local health departments across the United States are reporting an alarming surge in whooping cough, or pertussis, cases, a dramatic uptick in the respiratory disease that many had believed was in check. The Centers for Disease Control and Prevention (CDC) says there has been a staggering 1,500% increase in reported cases nationwide since 2021, fueling concerns of another widespread outbreak on top of measles.
The upward trajectory in the number of cases isn't just numeric, it's deadly. In Louisiana, two infant deaths have already been confirmed in 2025, the state's first pertussis deaths since 2018. In Texas, the Laredo Public Health Department has already tallied more cases this year than in all of 2024. North Carolina and Michigan are also in triple-digit outbreaks, with 166 and 520 cases, respectively.
In 2023, the U.S. recorded 7,063 cases of pertussis. That spiked to 35,435 in 2024, including 10 deaths—primarily infants under the age of one, reports the American Academy of Pediatrics. Stated Dr. Andrea Garcia, American Medical Association Vice President of Science, Medicine, and Public Health, "It's the highest number in more than a decade."
Historically, pre-pandemic years saw around 15,000–19,000 cases annually. The current spike is not just a statistical uptick, but a red flag for public health.
Why Is Whooping Cough So Dangerous?
Pertussis is a highly contagious respiratory bacterial infection caused by Bordetella pertussis. It infects the upper respiratory system by binding to cilia—microscopic hair-like appendages that function to clear airways—and producing toxins that kill these structures and lead to inflammation.
Whooping Cough Symptoms
The disease is especially dangerous for infants under one year of age, and approximately one out of three is hospitalized due to severe complications such as pneumonia, seizures, or apnea (life-threatening pauses in breathing).
Early symptoms are misleading: runny nose, low-grade fever, and a mild cough—so it's hard to rule out ordinary colds, COVID-19, or RSV. But within one to two weeks, the characteristic "whooping" cough appears, with violent coughing fits that sometimes conclude with a high-pitched "whoop" sound when breathing in.
The illness can last anywhere from 6 to 10 weeks, and while older children and adults may experience milder symptoms, they can still spread the infection to vulnerable populations.
How Long Is Whooping Cough Contagious?
One of the biggest questions for families and caregivers: How long is a pertussis victim contagious?
The patient can infect others as soon as symptoms of a cold appear and is contagious for up to 3 weeks after the onset of coughing.
If antibiotics such as azithromycin, clarithromycin, or erythromycin are given at an early phase, the patient typically is no longer contagious after 5 complete days of antibiotic therapy.
This prolonged period of contagion underscores the need for early detection and treatment—not just for the recovery of the patient but to break the chain of transmission.
What's Causing the Surge in Whooping Cough Cases?
One of the underlying reasons for the recent spike in cases is low vaccination rates, which public health officials say could jeopardize decades of progress.
Dr. Garcia and others blame the resurgence on populations with low or waning immunity, particularly where vaccine coverage has dropped. This not only includes children but also adults who are due for booster shots, as vaccine immunity wanes over time.
How to Stay Protected?
The most effective way to prevent whooping cough is through timely and complete vaccination. For infants and young children, the DTaP vaccine—protecting against diphtheria, tetanus, and pertussis—is administered at 2, 4, and 6 months of age, with booster doses given between 15 to 18 months and again at 4 to 6 years. As children grow into adolescence, a Tdap booster is recommended at ages 11 to 12.
Adults who have never received the Tdap should get a single dose, especially if they are in contact with infants. Pregnant women are advised to receive a Tdap booster during the third trimester of each pregnancy to pass on protective antibodies to their newborns, offering vital early immunity. Additionally, healthcare workers and international travelers—particularly those in close contact with infants or vulnerable individuals—should ensure their pertussis vaccinations are up to date to help curb the spread of this highly contagious illness.
In those who have been exposed to the infection, post-exposure prophylaxis with antibiotics will prevent the disease from developing. The household contact or close contact must be called by the local health provider for confirmation of pertussis.
The sharp increase in whooping cough infections is more than just a number, it's a public health warning sign. The disease, as preventable as it is, is killing infants and testing the limits of community immunity across the country. With its long infectious period, easily mistaken symptoms, and widening transmission rates, pertussis is a genuine threat, especially for infants.

Credits: Canva
Medical Cannabis May Help Fight Cancer, Largest-Ever Study Shows Potential To Treat Symptoms
The largest-ever scientific study on cannabis and cancer has shown strong evidence that medical cannabis can do more than treat symptoms — it could actually fight the disease itself. The research, published in Frontiers in Oncology, provides a robust and data-heavy analysis that brings much-needed clarity to a very contentious issue.
Led by Ryan Castle, Research Director of the Whole Health Oncology Institute, this revolutionary analysis converges more than 10,000 studies — the largest such inquiry into medical cannabis and cancer to date.
Cannabis has been in the middle of medical controversies and legislative wars for a long time. Traditionally, the Schedule I status of cannabis under federal law has limited high-quality human clinical studies, preventing the medical community from reaching a consensus. Castle and his colleagues aimed to change that.
Our aim was to establish the scientific consensus on the issue of medical cannabis, an area that has been long dominated by a war of cherrypicked studies," Castle said.
In order to transcend prejudice, Castle's team took the large-scale, inclusive approach fuelled by AI and sentiment analysis — a process widely employed by natural language processing to determine whether written material portrays a positive, neutral, or negative sentiment. Here, AI analyzed thousands of abstracts and conclusions drawn in scientific literature to determine whether each one stated agreement, neutrality, or doubt in cannabis's applicability to the treatment of cancer and symptom alleviation.
The result? An overwhelming majority of studies presented a positive view, indicating medical cannabis holds therapeutic value not just for symptom relief — such as reducing inflammation and nausea or boosting appetite — but potentially for accelerating apoptosis, the death of cancer cells.
Castle's group examined over 10 times the amount of research examined in any other meta-analysis. Their report stated that roughly 55% of studies indicated a positive relationship between medical cannabis and favorable cancer outcomes with only a few percent reporting adverse effects or none at all.
That percentage — 55% — may seem humble, but with the sheer scale of the data and the scientific conservative tradition in this area, it's telling. "This level of statistical consensus is precisely what we required to start thinking of cannabis as more than an edgy cure-all," Castle wrote.
In addition, the National Cancer Institute (NCI) revealed that 20% to 40% of patients with cancer currently use marijuana products to deal with side effects such as constant pain, chemotherapy-related nausea and vomiting, and sleeplessness. Still, investigations had fallen behind trend usage rates based on government policy and availability of funds in the past.
It is valuable to place the results of this mega-study in the context of the overall body of cannabis research. Much of what is published is derived from in vitro (test tube) experiments or animal models and not human trials. However, several of these studies have promising results: compounds found in cannabis — particularly cannabinoids such as delta-9 THC, CBD, and CBG — have been shown to inhibit cancer growth, prevent metastasis, and cause cancer cell death in laboratory experiments.
A 2023 Discover Oncology study supported this perspective, reaching the conclusion that multiple cannabinoids reveal "promising potential as anticancer agents by multiple mechanisms." These include curbing tumor expansion, inhibiting cancer cell invasion, and cutting inflammation — which is a documented driver of cancer growth.
In addition, more recent studies have discovered unforeseen advantages in cancer patients who use cannabis. A University of Colorado study reported that patients who consumed marijuana products from licensed dispensaries for a two-week period reported better thinking and cognition, contrary to previous concerns that cannabis would impair mental sharpness in chronic users.
Even with all the encouraging results, Castle and other specialists advise not to consider cannabis a panacea. The available evidence does not indicate that medical cannabis by itself can heal cancer. Rather, its actual strength is in integrative oncology — as an adjunct therapy in addition to standard therapies such as chemotherapy, radiation, and immunotherapy.
This is in agreement with results of a 2019 literature review, which noted that cannabis potentially slows cancer growth and aids improved treatment outcomes, although its effectiveness is highly variable based on cancer type, formulation of cannabinoids, and dose.
One of the significant challenges that persist in holding back development in this arena is the federal classification of cannabis as a Schedule I drug. According to existing U.S. policy, cannabis is listed alongside drugs such as heroin — a designation that presents many legal and bureaucratic hurdles to researchers and healthcare professionals.
In addition, in the Trump era, the National Cancer Institute highlighted marijuana as one of almost two dozen "controversial or high-profile issues" that needed extra clearance prior to publication or research sharing. This culture of fear and repression has only held back the much-needed research into medical cannabis's complete potential.
The biggest-ever analysis of medical cannabis and cancer isn't asserting to have discovered a cure but what they have discovered is a mounting scientific concurrence that medical cannabis should be included in mainstream discussions of cancer treatment. From reducing side effects to having the potential to interfere with cancer cell life cycles, cannabis might have more than just palliation to provide — it might have clinical benefit.

Dr. Mathew Kalarickal, 'The Father of Angioplasty' In India Passes Away At 77; How The Procedure Transformed Heart Care?
Dr. Mathew Samuel Kalarickal, a pioneer of interventional cardiology, has passed away on April 18, 2025, in Chennai, at the age of 77, marking an end to an era in coronary angioplasty and stenting technology. Popularly known as the 'Father of Angioplasty in India,' Dr. Kalarickal transformed heart care, changing the lives of thousands of patients and redefining the face of contemporary interventional cardiology.
Born on January 6, 1948, in Kerala, Dr. Kalarickal's journey to becoming one of the world's most renowned cardiologists was set early in life. After completing his medical studies at Kottayam Medical College, he went on to pursue specialization in cardiology from Chennai but his stint in the United States of America, under the guidance of Dr. Andreas Gruentzig—the man universally accepted as the 'Father of Coronary Angioplasty'—would establish the foundation for his groundbreaking career.
Dr. Kalarickal's return to India in 1985 proved to be turning point. Coronary angioplasty was a new, unexplored area in India at that time, and one that fell behind progress in the U.S. and Europe. Sensing this lacuna, Dr. Kalarickal chose to introduce this revolutionary procedure to India, with a vision of making lifesaving heart procedures reach more people.
First Angioplasty in India
In 1986, Dr. Kalarickal performed the very first angioplasty in India, a process which would subsequently alter the direction of heart treatment in the country. Angioplasty at that time was not a widely known procedure in India, and coronary artery disease was on the rise. During the first year, he had only treated 18 patients. But by 1987, that figure had risen to 150, an unmistakable indicator of both the increasing demand for this life-saving operation and the confidence that patients had in Dr. Kalarickal's skills.
His success in India did not remain confined to its borders, Dr. Kalarickal played a key role in setting up angioplasty centers in various nations in the Asia-Pacific region, such as Pakistan, Bangladesh, Sri Lanka, the United Arab Emirates, Indonesia, Thailand, and Malaysia. His relentless efforts to educate and train physicians in these nations helped ensure that this new technique spread like wildfire, eventually saving countless lives and making heart procedures more available worldwide.
Role of Dr. Kalarickal in Expanding Angioplasty Globally
Dr. Kalarickal's role was not just to bring angioplasty to India and the rest of the region. As an innovator, he was a pioneer in bringing new innovations to the world of angioplasty and stenting. One of his greatest feats was becoming the first Indian cardiologist to introduce and practice the application of drug-eluting bio-absorbable stents, which improved the efficacy of angioplasty by a large margin and minimized the threat of re-blockage in coronary arteries.
Having done more than 10,000 angioplasties, Dr. Kalarickal's expertise and commitment to improving heart health were second to none. He also contributed significantly to academia, establishing the National Angioplasty Registry of India, through which data on angioplasty operations could be gathered and analyzed to streamline and enhance practice nationwide. His contributions had an effect on the medical fraternity and made him a mentor to numerous budding cardiologists in India and overseas.
Dr. Kalarickal's success was not limited to the operating room. His leadership positions in major medical societies demonstrate his reputation as a world leader in interventional cardiology. He was president of the Asian-Pacific Society of Interventional Cardiology from 1995 to 1997 and then went on to chair the Asian-Pacific Society of Cardiology section of Interventional Cardiology between 1995 and 1999. His presidency in these societies promoted the use of angioplasty and stenting procedures around the world and consolidated the group of cardiologists in Asia.
His work was duly appreciated in many awards and honors. Dr. Kalarickal received the esteemed Padma Shri award in 2000, one of the highest civilian awards in India, for his outstanding work in cardiology. He was also awarded the Dr. B.C. Roy Award in 1996 for his notable contributions to medicine, the Doctor of Science Award by Dr. M.G.R. University in 2003, and a Lifetime Achievement Award in 2008.
While Dr. Kalarickal was well-known for his medical knowledge, he was as much admired for being a mentor. Dr. Sai Satish, who is a senior interventional cardiologist in Chennai, was trained by Dr. Kalarickal and collaborated with him for more than two decades. Talking about his experiences during his mentor's time, Dr. Satish stated, "There will never be another Dr. Mathew Samuel Kalarickal in my life.". He taught me in ways that few people ever managed, and I will miss him every time I enter a cath lab." This is a sentiment shared by many other cardiologists who were fortunate enough to learn from him. His dedication to educating and empowering the future generation of heart doctors has left an invaluable legacy on the specialty of cardiology.
Dr. Kalarickal's contributions have saved thousands of lives, and his legacy will never be lost in the profession of interventional cardiology. His vision, commitment, and pioneering attitude have revolutionized heart disease treatment in India and across the globe. With the advent of angioplasty, he revolutionized the procedure that used to be an extremely invasive and dangerous one and turned it into a routine, life-saving one.
How Angioplasty Saves Your Heart?
Angioplasty is a minimally invasive procedure to open up narrowed or blocked coronary arteries due to a buildup of fatty plaques. Plaque buildup in the arteries over time can limit blood supply to the heart, resulting in angina (chest pain) or even heart attacks. Angioplasty is done to relieve these blockages, restore normal blood flow, and prevent heart-related complications. But in what way precisely does angioplasty save the heart?
While undergoing angioplasty, a catheter is inserted into the clogged artery with a balloon at the end. The catheter is advanced through the bloodstream with great caution until it enters the area where the blockage is. Having reached its destination, the balloon is inflated, pushing the plaque against the artery walls, effectively opening up the artery and reinstating blood circulation to the heart. In most instances, a tiny mesh tube known as a stent is also inserted to keep the artery open and prevent it from closing again.
Angioplasty can be carried out in patients with different types of coronary artery disease, such as patients who have experienced heart attacks, those with chronic angina, and those at high risk for cardiac events due to plaque deposition. Angioplasty is commonly carried out on patients who are not ideal candidates for standard open-heart surgery.
Instant Relief and Long-term Advantages
Perhaps the greatest benefit of angioplasty is the relief that it brings promptly to the patient. Post-procedure, patients can notice dramatically decreased symptoms of chest pain, shortness of breath, and tiredness, all typical with clogged arteries. The normalization of blood flow tends to keep the heart from working as hard and lowers the risk of heart attacks.
In the long run, angioplasty ensures that the heart is not subjected to further harm by providing it with a sufficient supply of oxygenated blood. This is particularly vital in individuals suffering from coronary artery disease because continuous blood flow is crucial to maintaining heart muscle health. Angioplasty can greatly eliminate the risk of heart failure, heart attack, or stroke by opening up clogged arteries. Also, the procedure has been demonstrated to enhance quality of life in general, since patients are frequently able to resume normal activities following the procedure without the restrictions created by chest pain and other symptoms.
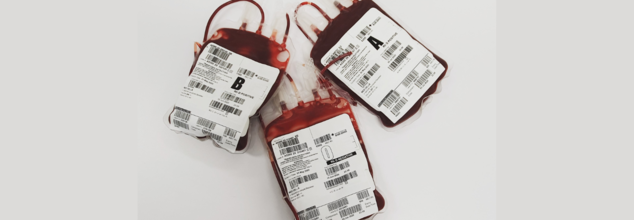
Credits: Canva
Scientists Discover Gut Enzyme That Converts Blood Type A & B Into 'Universal Donor' Type O
Scientists at the University of British Columbia (UBC) have discovered a gut bacterium that may transform blood transfusions across the globe. The bacterium, Akkermansia muciniphila, found naturally in the human gut, secretes enzymes that can convert blood types A, B, and AB to type O—the universal donor type. The discovery, reported in Nature Microbiology, may substantially resolve global blood shortages and enhance transfusion success in critical care situations.
Compatibility in blood groups is crucial in transfusions. Red blood cells of ours have antigens—specific sugar-protein molecules—on their surface. These antigens define our blood type (A, B, AB, or O). O blood cells don't have A and B antigens, and therefore, these are compatible for patients with A+, B+, AB+, or O+ blood. This is why type O, particularly O-negative, is the most sought-after blood group in emergency and trauma centers across the world.
However, blood donations usually don't meet the required demand. This is a serious problem because, while more than 117 million pints of blood are donated every year globally, still, there are mismatched types in both planned and emergency operations. If there were a safe and scalable way of converting A, B, and AB blood into O, healthcare systems would be revolutionized and thousands of lives could be saved.
The secret to this change is in the enzymes secreted by Akkermansia muciniphila. These enzymes hydrolyze mucin, a glycoprotein rich in sugar that lines and protects the gut. Interestingly, the structure of mucin is similar to the sugar chains present in blood group antigens.
Working with donor samples, UBC scientists extracted a particular pair of enzymes from the gut flora of an AB+ donor. These enzymes effectively broke down A and B antigens on red blood cells, essentially turning them into type O universal donor cells. The researchers even went on to work with a complete unit of A-type blood using this technology—a milestone never reached before using this level of efficiency and safety.
A key component of this finding was cracking the code for the structure of the new enzyme by using crystallography. The researchers collaborated with the Canadian Light Source (CLS) at the University of Saskatchewan to see the structure of the enzyme, which enabled them to know why it had such high affinity for A antigens and why it was more efficient and quicker than other enzymes utilized in similar endeavors before.
The enzymes performed impressively well, even on the recently-discovered longer A and B blood group variants—a feat that earlier conversion methods faltered at. Interestingly, the conversion of blood type B was more straightforward than that of type A, though both could be successfully treated under room temperature conditions in only 30 minutes, and without chemicals—a quantum leap in rendering the process viable for the clinic.
What is ECO Blood?
The scientists refer to the transformed samples as "enzyme-converted to O" or ECO blood. It takes only around 8 grams of the enzyme to convert 200ml of blood, indicating the process can be scaled up for practical medical application. In contrast to previous techniques first tried in 2007, which were hampered by efficiency and safety issues, this new technique is faster, cleaner, and more compatible with hospital environments.
ECHO blood is a term for echocardiography, a non-invasive medical imaging test that uses high-frequency sound waves to create detailed images of the heart in real-time. The imaging method enables physicians to evaluate the functioning of the heart by viewing its size, shape, pumping capability, and blood flow through the heart's chambers and valves. Echocardiography is important for diagnosing heart disease, heart health monitoring, and making decisions about treatment without surgery or the use of radiation.
These results are timely, as over half the population in nations such as Canada will require blood or have someone who does during their lifetime. Furthermore, shortages of blood are a worldwide problem, frequently compounded by emergencies, natural disasters, and pandemics that disrupt donation cycles. If effective in human trials and cleared for clinical use, this enzyme technology may become a pillar of contemporary transfusion medicine.
Although the discovery is a breakthrough, the scientists are being cautious. Although the type B blood conversion has been done effectively, further work is required to convert type A blood reliably and safely in all subtypes. Future research will aim to optimize these enzymatic reactions and scale up the process for clinical trials.
Lead researcher Dr. Stephen Withers pointed out the clinical significance, "Our conversion of RBCs was particularly conducted at the maximum red blood cell concentration, minimum assay time, and minimum temperature compared to existing literature. The conditions are important in terms of keeping it mild enough for use clinically.
This breakthrough illustrates the enormous potential of microbiome research to address some of medicine's most enduring challenges. By tapping into the untapped potential of gut bacteria, scientists could soon be eradicating blood type barriers—opening up a future in which safe, universal blood transfusions are no longer confined by compatibility.
How It Works?
Blood type is assigned by particular antigens—carbohydrate-based molecules—found on the surface of red blood cells. Type A people have A antigens, type B people have B antigens, and AB people have both. Type O people lack both A and B antigens, so their red blood cells are compatible for transfusion across the board, particularly in crisis situations when matching bloods might not be immediately on hand.
The enzymes from A. muciniphila effectively "shave off" the A and B antigens from red blood cells, reducing them to a neutral, antigen-free state—Type O. In the laboratory, scientists successfully treated Type A red blood cells with this enzymatic cocktail, stripping away the antigens effectively and within a relatively short time period of 30 minutes at room temperature. They also achieved similar results with Type B blood, and even with extended variants of A and B groups recently discovered.
Practically, around 8 grams of this enzyme blend can be used to convert 200 milliliters of A or B blood into "enzyme-converted to O" (ECO) blood. This conversion was achieved at high red blood cell concentration, under mild and additive-free conditions, which are best for clinical feasibility and safety of the patient.
How Does This Breakthrough Affect Healthcare?
The capacity to transform types A and B of blood into universal donor type O is a giant step in the history of transfusion medicine. Blood transfusions are salvage operations employed during surgery, traumatic injury, cancer therapy, and chronic disease, but they are reliant on blood group compatibility to prevent potentially lethal immune reactions. The major hindrance in emergency care and blood bankation is the scarcity of type O blood, which is the universal donor for Rhesus (Rh) factor positive individuals—representing close to 75% of the world's population.
Through the use of gut-derived enzymes from Akkermansia muciniphila to remove antigens from A and B red blood cells, scientists have created a new avenue for increasing the availability of universal blood. This could mean hospitals need to use donors and recipients less often based on matching by blood type, a process that all too frequently causes delay in such procedures, particularly in emergencies. It also would mean that short supplies or unusual blood types might be reduced through converting more typically available types into type O.
At a wider health care level, this innovation has the potential to ease the global shortage of blood donors, improve blood bank inventory management, and provide greater availability of blood in rural or under-resourced areas where certain types are difficult to obtain.
© 2024 Bennett, Coleman & Company Limited

