- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories

Credits: Canva
Telangana Reports A Surge In Obesity, Anxiety And Other Lifestyle Diseases
A new report released by the Apollo Hospitals on Monday, which has raised concerns about the state of health in Telangana. The report is titled Health of the Nation 2025 (HoN-2025). this is the 5th edition of this study, which is based on health screenings of over 44,000 individuals across seven Apollo centers in Telangana. The findings present a worrisome state of Telangana. The issues that raise concerns are mostly lifestyle diseases and disorders, including obesity, hypertension, diabetes, and mental health issues. The numbers show that all such health issues are on rise.
Obesity, Hypertension, and Diabetes On The Rise
One of the most striking findings from the report is that 63% of people who were screened were found to be obese. 19% of the people were also found to be overweight. This means that more than 4 in 5 individuals are carrying excess weight. This is concerning as obesity could be a risk factor for several chronic conditions including heart disease and diabetes.
The findings on blood pressure is also concerning. 23% of those who have been screened had hypertension or had high blood pressure. A much larger number, which is 55% were pre-hypertensive. This suggested a dangerous high number of people who are at risk of developing full blown hypertension in the near future.
The diabetes numbers are also worrying. Of the nearly 41,000 people screened, 25% were diabetic, while 34% were pre-diabetic. This meant that almost 60% showed elevated blood sugar levels.
How Was The Data Collated?
The data was drawn from Apollo centres in Miryalaguda, Warangal, Karimnagar, Secunderabad, DRDO, Hyderguda and Jubilee Hills. Apollo Hospitals used anonymised electronic medical records from preventive health checks, clinical evaluations, and AI-based risk analysis tools.
Mental and nutritional health needs urgent attention too
The report didn’t just focus on physical ailments—it also looked at mental health and nutrition. Among 20,612 people screened for mental health, 3% were diagnosed with depression, and a similar percentage showed symptoms of anxiety. While the percentages may seem small, they highlight growing mental health concerns that often go unnoticed or untreated.
On the nutritional front, the numbers are equally stark. 24% of those screened were anaemic, and an overwhelming 82% had Vitamin D deficiency. These deficiencies can lead to fatigue, weakened immunity, and long-term health problems.
Sleep health also came under scrutiny. Among over 20,000 individuals screened for Obstructive Sleep Apnea, 22% were found to be at risk. Sleep apnea can significantly affect quality of life and is linked to several cardiovascular and metabolic disorders.
Call for urgent action
Reacting to the findings, Preetha Reddy, Executive Vice-Chairperson of Apollo Hospitals, stressed the need for immediate intervention. “The report underscores the increasing rates of obesity and pre-hypertension, which is deeply concerning. These are not just statistics but signs that we must act swiftly,” she said.
With lifestyle diseases continuing to rise, the report makes it clear that public awareness, timely screening, and preventive care are more crucial than ever for Telangana’s population.

Credits: Instagram/ @drcaseyskitchen
Trump’s Surgeon General Pick Raises Eyebrows With Vaccine Comments And Wellness Claims
In another news, President Donald Trump's latest nominee for US surgeon general, Dr Casey Means, is better known to some as a rising wellness influencer. Over the past year, she has shared her health philosophy with many on various podcast channels, which are popular for wellness content, most of them being right-wing podcast, as US News describes it. These podcasts have discussed everything from organic diets, chronic diseases, their prevention, and of course, vaccine skepticism, and spirituality.
She may use the surgeon general's platform to promote the lifestyle-based approaches that she has been talking about, which may challenge the conventional views on pharmaceuticals, vaccines, and modern medicines.
Focusing On Root Cause
Now 37, Dr Means left her residency program to focus on what she calls the "root causes" of disease. She believes that chronic illnesses such as obesity, infertility, cancer, and Alzheimer's are not being properly addressed by traditional medicines.
As per her, a combination of environmental toxins, processed food, and tech overuse is damaging the body's metabolic system, which remains the core of most chronic health problems.
Speaking to Joe Rogan in October, she said: “It’s all caused by metabolic dysfunction, a term that I never learned in medical school.” Her philosophy closely mirrors that of Health Secretary Robert Kennedy Jr., who has called for deep investigations into environmental factors behind conditions like autism.
Dr. Means has voiced concern over the widespread use of certain drugs. On “The Tucker Carlson Show,” she criticized the overprescription of birth control pills, calling it a “disrespect of things that create life.” She also raised alarms over the use of popular weight-loss drugs like Ozempic, calling them “very dark” and suggesting they promote the false idea of a “magic pill” for health.
Rejection Of Processed Food
Much of Dr. Means’ health advice centers on diet. She advocates for organic, non-GMO foods and warns against refined sugars, pesticides, and ultra-processed meals. On Jay Shetty’s podcast, she even suggested an executive order to eliminate added sugars from school lunches.
Her concerns extend to seed oils—like canola and soybean—which she groups with harmful food additives. Although mainstream nutrition experts say such oils can be beneficial in moderation, Means believes they contribute to declining public health.
While Dr. Means often prefaces her vaccine comments by noting it's not her area of expertise, she has publicly questioned parts of the U.S. childhood vaccination schedule. On Kristin Cavallari’s podcast, she pointed to the hepatitis B vaccine for newborns as her “gateway” to questioning broader vaccine policies.
She also criticized COVID-19 vaccine mandates, saying they caused significant societal harm and sparked public distrust in health institutions. “Maybe we shouldn’t be blindly trusting the experts,” she said.
Dr. Means blends science with spirituality in her view of wellness. She’s described current public health trends as “extinction-level” and frequently says “Rome is burning” to describe the urgency. For her, healing requires not just lifestyle changes but also a deep spiritual reconnection with the body, Earth, and the divine.
“Do we want to connect with God and respect these temples we’ve been given?” she asked Rogan. “That’s the choice we have right now.”

Credits: Canva
New Gel-Based Antibiotics Could Carry Drugs To Hard-To-Reach Parts Of Ear Infection
Anyone who has ever cared for a child with painful ear infection would know how difficult those days and nights are when the child is unable to be at peace. Relief often takes time, and sometimes doe not at all come. However, ear infections in toddlers are quite a common phenomenon, and are often stubborn. Children can also develop resistance to standard antibiotics due to its constant use. This means the infection can return even after days of treatment.
So, what is the solution?
Researchers at Cornell University may have found it. There is an alternative that exist- a single-dose, topical antibiotic gel that could simplify treatment and reduce recuring infections.
What Does It Do?
While earlier, the doctors have relief on oral antibiotics, which also had side effects like upset stomachs, and year infections. This new method delivers medicines directly in the infected ear. Doctors predict this to be game changer, especially for children who struggle with oral medication.
However, the challenge is that most ear infections affect the middle ear, which sits behind the eardrum. This is the barrier that most drugs cannot cross. This is where the new approach and its innovation comes into play.
The Gel And The Science Behind It
The research is led by Rong Yang, assistant professor of chemical and biomolecular engineering, and the Cornell team have found their way around. They have packaged the antibiotic ciprofloxacin into microscopic delivery vehicles called liposomes. These are tiny, bubble-like structure which are used to carry drugs to hard-to-reach parts of the body.
These liposomes are negatively charged, which then helps them to bind better to the ear tissue. They are then incorporated into a gel-like salve, which is then applied directly to the eardrum.
What Do We Know Of The Trials?
In lab tests on chinchillas — whose ears closely resemble human ears — the results were impressive. The infections cleared up within 24 hours of a single application. Over the next week, no signs of the infection returned, and there was no inflammation in the eardrums.
The study, recently published in ACS Nano, marks a significant step toward making ear infection treatment faster, more effective, and less taxing on children and their families.
Are There Any Challenges?
Despite these promising results, researchers caution that what works in animals doesn’t always translate directly to humans. Other versions of the gel tested on chinchillas were less effective, and much more work remains before this treatment can be approved for children.
Still, the potential is real. "A single-dose treatment for middle ear infections represents a significant step forward," Yang said in a statement. "It could reduce the burden on families and improve outcomes for young children."
She added that moving from lab tests to clinical trials is the next key step: "It has the potential to improve patient compliance, reduce antibiotic resistance, and ultimately transform how children receive antibiotics."
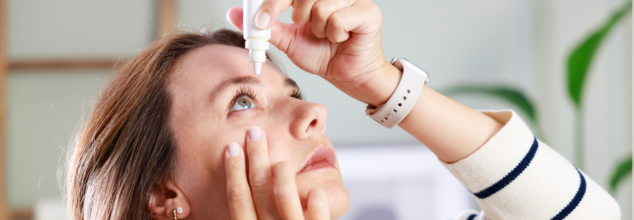
Credits: Canva
Over 2 Million Eye Drops Recalled In U.S. Amid Alarming Risk Of Blindness And Irreversible Damage
A massive recall has been issued for over 2 million eye drop cartons due to concerns over sterility that can be potentially harmful and lead to serious eye infections, blindness, and even permanent blindness in extreme cases.
The voluntary recall, issued jointly by AvKARE, a Tennessee pharmaceutical distributor, and the U.S. Food and Drug Administration (FDA), is being labeled a Class II recall—meaning that although the danger of serious harm is relatively minor, the harm can be transient or medically reversible.
The eye drops were recalled from store shelves after an FDA audit uncovered manufacturing deficiencies that rendered product sterility in doubt. Although neither the FDA nor AvKARE would reveal the specifics regarding the manufacturing deficiencies, the notice of recall mentioned a "lack of assurance of sterility"—a rather disturbing red flag for products that are applied directly to the eyes.
Ophthalmic sterility is non-negotiable. A compromise of sterility, however slight, invites bacterial contamination, which has the potential to penetrate the soft tissues of the eye and develop catastrophic infections.
These products have been shipped between May 26, 2023, and April 21, 2025, which could have endangered millions of customers—particularly those who are addressing chronic dry eye symptoms or season allergies.
How Can Eye Drops Cause Blindness?
To the typical consumer, a contaminated eye drop bottle may not appear to be anything. But the reality is darker. Non-sterile solutions can be a portal of entry for bacteria, fungi, or even parasites, all of which can wreak havoc on the internal environment of the eye.
In 2023, when contaminated eye drops containing a drug-resistant bacteria strain caused 50 infections in 11 U.S. states. That outbreak caused one death and several cases of permanent blindness, highlighting just how perilous contaminated eye drops are.
More disturbing is the likelihood that consumers will unknowingly use these recalled products, particularly at the height of allergy season, when dry eyes are a frequent complaint and over-the-counter relief is in high demand.
If you have recently used one of the recalled eye drops, beware of these warning signs of potential infection:
- Redness or swelling of the eye
- Pain or stinging
- Blurred vision or visual changes
- Abnormal discharge or watery eyes
- Increased sensitivity to light
Although no health problems have yet been officially reported regarding this particular recall, an early intervention is always the best course of action. If infection is suspected, stop using at once and seek an ophthalmologist. AvKARE has put out a strong notice:
Immediately stop using the recalled products. Retailers are requested to pull the products from store shelves and return all unsold merchandise to the distributor for a full refund, including the cost of return shipping.
The recall was initiated by BRS Analytical Service, LLC, an independent laboratory that tests pharmaceuticals for compliance. The action demonstrates growing scrutiny from the FDA, particularly after last year's death from eye drops.
Although the identity of the manufacturer is not revealed, the FDA has not eliminated further action, particularly if more products prove to be dangerous.
What This Means For Your Eye Health?
This event brings to the fore the larger issues regarding the regulation and testing of over-the-counter (OTC) eye care products. It acts as a reminder for consumers and medical professionals alike to ensure that proper eye hygiene, product handling, and consultation with medical practitioners at the right time are ensured.
Even synthetic tears, deemed by most to be harmless, may induce temporary blurring of the vision, allergic reactions, or introduce irritants in the form of preservatives or thickeners if improperly used. No prescription or OTC eye drop should be employed for longer than recommended, and sharing usage is strictly avoided.
The patient should also understand that improper packaging and expired usage can weaken the product and even inflict more harm. Always examine the seal and expiration date and never use bottles with broken or tampered packages.
What To Do If Your Eyes Are Affected?
You may see a complete list of the products recalled and lot numbers on the FDA or AvKARE's official notice of recall webpage. If you already have the affected eye drops:
- Discontinue use immediately.
- Place them in a sealed bag to avoid accidental opening.
- Get a refund or disposal guidance from your retailer.
- Consult a healthcare professional if you're experiencing symptoms.
Medical practitioners are also cautioned to inform the FDA's MedWatch Safety Information and Adverse Event Reporting Program about any product quality issues or adverse events.
Stay up to date, read labels attentively, and never settle for the safety of what you put in your eyes. If you use eye drops frequently, think about talking to a certified ophthalmologist to discuss preservative-free options and individualized choices that are safer for long-term use.
© 2024 Bennett, Coleman & Company Limited



