
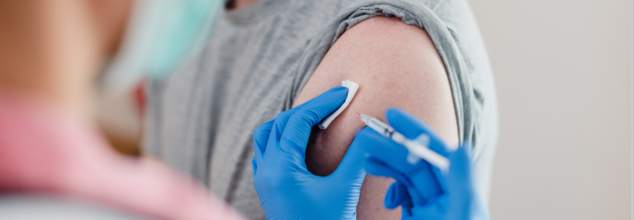
Smoking Could Lead To Nearly 300,000 Cancer Cases In The UK By 2029, Claims New Study
Smoking Could Lead To Nearly 300,000 Cancer Cases In The UK By 2029, Claims New Study
Smoking remains a major public health challenge in the UK, with a new study by Cancer Research UK predicting that tobacco use could cause nearly 300,000 new cancer cases by 2029. This stark finding has prompted urgent calls for lawmakers to support the proposed tobacco and vapes bill, which aims to eradicate smoking in the country by introducing age-based restrictions on tobacco sales.
The research, highlights the devastating impact of smoking on public health. If current smoking trends continue, then by the end of this parliamentary term in July 2029, there are chances that the UK might record 296,661 new cancer cases attributable to tobacco. The regional breakdown stands at an estimated 243,045 in England, 29,365 in Scotland, 15,161 in Wales, and 9,090 in Northern Ireland.
Alarmingly, it might cause an estimated 2,846 added cancer cases in non-smokers. On average, almost 160 smoking-related cancer cases were diagnosed daily in 2023, reflecting the continued burden of tobacco on the nation's health care system.
Given this, Cancer Research UK is calling upon MPs to vote to pass the tobacco and vapes bill scheduled for its second reading in the House of Commons. Proposed to make the country among the first in the world to eradicate smoking with a proposed legal age to buy tobacco products, from which smoking would effectively be prohibited for everyone born on or after 1 January 2009.
According to charity, this bill is a "historic opportunity" in saving lives, preventing thousands of new cancer cases, and reducing the enormous burden on the NHS, as well. Smoking has remained the leading cause of preventable deaths in the UK, and since about 350 young people start smoking each day, actions must be taken speedily.
Effect of Smoking on Youths
Smoking remains a popular habit among young adults, despite its prevalence falling. In 2023, an estimated 11.9% of those aged over 18, or around 6 million people, smoked cigarettes; this is the lowest proportion since these records started in 2011. However, according to Cancer Research UK, 35,000 young adults aged between 18 and 25 have started smoking since July.
Such a trend draws to close attention an urgent need for targeted measures to prevent initiation of smoking among the youth. The bill seeks to include restrictions on vape advertisements and sponsorships, flavorings, and packaging, not to mention a complete ban on smoking and vaping in playgrounds and close to schools.
Cancer Research UK argued that the "scale of harm" caused by smoking is now too big to be ignored. In this context, the charity saw the bill on tobacco and vapes as an important step toward a smoke-free generation with reduced preventable burden of diseases caused by tobacco.
Every day, 350 young people are enticed into smoking-a habit that may lead to a life of health complications," a charity spokesperson said. "This law provides the opportunity to reverse this trend and shield future generations from the catastrophic effects of tobacco.
As MPs debate the bill, there is little doubt of what is at stake: without major action now, the costs of tobacco on public health will continue to escalate. The new legislation is not merely preventive in its intent; it is a promise of protection for millions and the enormous relief on healthcare from smoking.

Credits: Canva and Gilbert Carrasquillo
Against Experts' Concerns, HHS Releases A New Autism Study
The US Department of Health and Human Services (HHS) is now preparing a large-scale research initiative which will look at understanding the causes of autism. This announcement came from Secretary, Robert F Kennedy Jr. on Thursday after a Cabinet meeting with President Donald Trump.
What Will This New Research Do?
According to Kennedy, the HHS project will be a “massive testing and research effort” involving hundreds of scientists, with the goal of completing the work by September. The effort, however, has sparked immediate concern among scientists, autism advocacy groups, and public health experts, particularly because of Kennedy’s long-standing belief in a debunked theory linking vaccines to autism.
“There’s got to be something artificial out there that’s doing this,” President Trump told Kennedy during the meeting. “If you can come up with that answer, where you stop taking something, eating something, or maybe it’s a shot. But something’s causing it.”
RFK Controversy On Autism And Vaccine
Kennedy's earlier remarks and Trump's comments have already been taken with a pinch of salt, especially when they tried to revive the widely discredited claim that childhood vaccines could in fact cause autism. This theory originated in a 1998 study published in The Lancet, which falsely linked the MMR (measles, mumps, and rubella) vaccine to autism. The study was later retracted due to unethical practices and flawed data. Its lead author lost his medical license.
Despite overwhelming evidence disproving the vaccine-autism link, Kennedy has continued to promote it. During the Cabinet meeting, he restated his position:
“I saw my children get sick after getting vaccinated, and I’ve heard thousands of parents say the same. It’s time we take a serious look. This study is going to find out if something we’re doing—maybe the vaccines—is behind the autism epidemic.”
His statement was met with mixed reactions, especially from medical experts and autism advocates.
What Does Science Really Say?
Decades of research have failed to show any credible link between vaccines and autism. Major public health institutions—including the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), the American Academy of Pediatrics, and advocacy groups like Autism Speaks—have all concluded that vaccines do not cause autism.
Autism, a developmental condition affecting communication, behavior, and learning, has more scientifically established risk factors. These include:
- Genetic influences, which are the most significant contributors
- Environmental exposures such as air pollution and pesticides
- Low birth weight or premature birth
- Parental age, with older parents being more likely to have children with autism
The National Institutes of Health (NIH) already invests over $300 million annually in autism research, focusing on early detection, interventions, and understanding underlying causes. It remains unclear how Kennedy’s study will differ or whether it will duplicate ongoing efforts.
What Do The Experts Say?
The Autism Society of America and other groups have raised concerns over the new initiative. “There is a deep concern that we are going backward and evaluating debunked theories,” said Kristyn Roth, a spokesperson for the Autism Society. She also noted that their organization had not been consulted in any part of the study’s planning.
Another source of controversy is Kennedy’s choice to appoint Dr. David Geier, an orthopedic surgeon who has long claimed a link between vaccines and autism, to lead the study. In 2011, Maryland authorities found Geier was practicing medicine without a license.

Credits: Canva
Alabama Woman Lives 130 Days with Pig Kidney, Undergoes Removal After Organ Rejection
In one of the most significant moments in the history of xenotransplantation, Towana Looney, a 54-year-old woman from Alabama became the first human to live for 130 days with a gene-edited pig kidney. Her journey has now ended with the organ's removal, but it yet remains an essential landmark in the field of medicine, especially in the search for viable animal-to-human transplants.
What Actually Happened That Led To The Xenotransplantation?
Towana Looney underwent the groundbreaking surgery on November 25, 2023, at NYU Langone Health. She had been on dialysis since 2016 and was ineligible for a human kidney transplant due to her immune system being highly sensitized, meaning it would likely reject a human organ. As a result, she volunteered to participate in an experimental xenotransplant procedure using a genetically modified pig kidney.
The transplant initially worked well. Looney even referred to herself as “superwoman,” noting that she felt better than she had in years. However, on April 4, 2024, after more than four months, her body began rejecting the organ, and doctors decided to remove it. She is now back on dialysis and recovering at home in Gadsden, Alabama.
Why Was The Pig Kidney Removed?
The decision made to remove the kidney came from the safety concerns as she developed an infection, which had been tied to her earlier dialysis treatment. It required doctors to reduce her immune-suppressing medicines. Around the same time, her immune system began reactivating, possibly triggering the rejection. Rather than risking a stronger dose of anti-rejection drugs—which could have caused further complications—her doctors chose to remove the kidney.
“We did the safe thing,” said Dr. Robert Montgomery, Looney’s surgeon and a leading figure in xenotransplantation. He emphasized that Looney wasn’t worse off than she had been before the transplant and, in fact, had benefited from 130 days free of dialysis.
ALSO READ: After Pig Kidney Transplants, Scientists May Have Found A Way To Transplant Pig Livers
What Is Xenotransplantation?
Xenotransplantation is the process of transplanting organs or tissues between different species—in this case, from pigs to humans. Scientists are modifying pigs so their organs resemble human ones more closely, in hopes of addressing the severe shortage of donor organs.
Currently, more than 100,000 people in the U.S. are waiting for organ transplants, the majority needing kidneys. Thousands die every year waiting. If successful, pig organs could help close this gap.
Looney’s case is part of a broader effort to test pig organ viability in people who are not terminally ill. Previously, four other U.S. patients had received pig organs—two hearts and two kidneys—but none of those organs lasted longer than two months, and all recipients eventually died due to pre-existing health issues.
What Is Going To Happen Next?
Despite the setback, researchers are hopeful. In fact, the news is that a man in New Hampshire who received a pig kidney in January is doing well. More clinical trials are also expected to begin this summer to give a better answer on where we stand with xenotransplantation. Chinese researchers have also reported success with a recent pig kidney transplant.
One of the major ongoing challenges is determining the right combination of immune-suppressing drugs to prevent different types of organ rejection without increasing infection risk. As Dr. Tatsuo Kawai from Massachusetts General Hospital noted, “When we have more experience, we’ll know what kind of immunosuppression is really necessary for xenotransplant.”
Credits: Canva
Flu Shot Linked To Higher Risk Of Infection This Year, Study Raises Eyebrows- Should You Be Concerned?
This flu season has turned out to be quite a surprise. A new study from Cleveland Clinic indicates the 2024–2025 flu shot might have fallen short — and potentially worsened the situation. With vaccinated patients reportedly having higher infection rates, health professionals and the general public are now reassessing the vaccine's effectiveness in real life.
New research from the Cleveland Clinic has caused alarm among health professionals and the general public. In contrast to years of conventional wisdom, the current flu shot may not only have been useless — it could actually have made some people more susceptible to the flu.
Released as a preprint on MedRxiv.org, the Cleveland Clinic study compared infection rates among more than 53,000 of its own staff and concluded that vaccinated staff had a higher rate of flu infections than their unvaccinated peers. Although the study is not yet subject to peer review, early conclusions have sparked a firestorm of questions about vaccine effectiveness, public health policy, and efforts to prevent future influenza.
Carried out across 25 weeks of the 2024-2025 respiratory viral season, the study monitored flu infection rates among Cleveland Clinic staff — predominantly a cohort of healthy adults of working age.
- Vaccinated staff had a 27% higher rate of flu infections than the unvaccinated cohort.
- Effectiveness of the vaccine was estimated at -26.9%, reflecting not only diminished protection, but a seeming increase in risk.
- The rate of influenza initially tracked similarly in both groups, but increased more steeply in the vaccinated as the season went on.
Notably, almost 99% of the vaccinated workers got the trivalent inactivated influenza vaccine, so it is difficult to know whether other formulations of the vaccine might have produced different results.
What Does "Negative Vaccine Effectiveness" Really Mean?
Negative vaccine effectiveness — particularly in the range of -26.9% — is a dramatic and disconcerting statistic. Yet, it does not indicate the vaccine induces the flu. Rather, it suggests that in this specific season, the vaccine provided no significant protection and potentially was not well matched to the prevailing virus strains.
The Cleveland Clinic explained in a statement to Fox News Digital, "The results do not suggest that vaccination increases the risk of flu. Instead, the study implies that the effectiveness of this season's vaccine in preventing influenza may have been limited in relatively healthy healthcare workers."
That is to say, the virus could have caught up with the shot.
While the results are surprising, specialists point out that this study is far from being the last word regarding the effectiveness of flu shots. There were several limitations the authors have acknowledged:
The research did not test flu severity of symptoms, hospitalizations, or fatalities — which are important indicators of vaccine worth.
It tested only healthcare workers, but not children, elderly people, and immunocompromised individuals, who most usually benefit from flu vaccination.
The vast majority of participants were given one type of vaccine, so the performance of others remains unknown.
Dependence on home test kits could have resulted in underreporting or misclassification of certain cases.
Why the Flu Shot Works Differently Each Year?
Performance of flu vaccines differs from year to year because of a number of factors:
Strain Mismatch: Every year, vaccine manufacturers make an educated estimate of the most common flu strains. If they're incorrect, the vaccine will provide suboptimal protection.
Population Immunity: Immune response varies with age, health, and past experience with flu viruses or vaccines.
Mutation Rates: Flu viruses change rapidly. Even when production starts, strains can change, lowering efficacy.
This year could just have been an anomaly in terms of immune response or strain drift — but the stakes are high for how we handle flu vaccination in the future.
Physicians warn not to make quick judgments based on a single preprint study. Dr. [Name], infectious disease physician at [Institution], cautions:
While Cleveland Clinic research is definitely worth further examination, it's important to keep in mind that the flu vaccine's greatest aim is to curb severe illness and mortality. We just don't have enough data yet to realize the total effect of this year's vaccine.
The Centers for Disease Control and Prevention (CDC) still encourages yearly flu vaccination for all people six months and older based on long-standing proof that flu vaccinations decrease the possibility of serious complications from the flu.
Should You Still Get the Flu Shot?
The quick answer: Yes — but with awareness.
This study points out that flu vaccine effectiveness can vary, and in extreme cases, may even not protect as expected. But overall evidence still continues to affirm the vaccine's function of reducing hospitalizations, decreasing duration of illness, and safeguarding high-risk groups.
If anything, the results emphasize the importance of ongoing investment in the development of vaccines, improved real-time surveillance of viral mutations, and open reporting of vaccine performance.
The Cleveland Clinic study doesn't mark the end of the flu shot — but it does pose important questions about how we produce, test, and present vaccine effectiveness. As more evidence comes in and peer-reviewed evaluation is finalized, health authorities will most likely fine-tune their approach to prevent future flu seasons from catching us unprepared.
© 2024 Bennett, Coleman & Company Limited

