- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
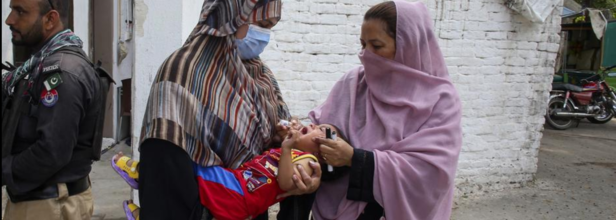
Polio Outbreak In Pakistan: 37 Cases Confirmed As Health Officials Call For Action- Should India Be Worried
Polio Outbreak in Pakistan
Pakistan continues to be dealing with a polio outbreak as four fresh cases have emerged, pushing the national tally to 37 this year, according to health officials on October 19, 2024. Health officials said that the regional reference laboratory for polio eradication at the National Institute of Health in Islamabad confirmed wild poliovirus type-1 (WPV1) in two children-one from each Balochistan and Khyber-Pakhtunkhwa.
In recent cases, a girl has been affected from Pishin, and two boys from Chaman and Noshki of Balochistan, and a girl from Lakki Marwat in KP. These are the first detections of the virus within Noshki and Lakki Marwat this year; isolated cases of poliovirus were previously reported within Chaman and Pishin. The province of Balochistan was the worst hit with 20 cases, Sindh had 10, Khyber Pakhtunkhwa had five, and Punjab and Islamabad had one case each.
A gigantic fight against polio has been on going in Pakistan- especially in Balochistan and southern KP-over the last two years. Immunisation campaigns have often been suspended or delayed because of local protests, insecurity, and community boycotts. Consequently, quite a number of children did not get the necessary vaccinations, making existing patches of vulnerability for the virus to flow within those pockets.
Noshki, located near Afghanistan's border, and Lakki Marwat have also recently reported some positive environmental samples that confirm the virus is present here, said a local reference laboratory official. Samples of latest cases are currently under genetic sequencing for checking spread of virus and origin.
As the threat of polio continues to grow, Pakistan has vowed to mount a nationwide campaign against it beginning from October 28. With the zeal to tackle the menace in the most effective manner, over 45 million children under the age of five will be vaccinated across the country.
Today, Afghanistan and Pakistan remain one of the few countries where polio has not yet been eradicated. The WHO said the virus remains a potential serious public health threat in areas with low vaccination coverage and weak surveillance.
India On Alert Against Polio
The country declared itself polio-free since 2014 and has kept the disease on bay almost a decade with very robust vaccination programs; however, two cases of vaccine-derived poliovirus cases reported in recent days from Meghalaya create some amount of doubts over a possible resurgence. Experts observe that in India, despite these detections taking place, strong coverage of vaccination at 90-95% and mandatory surveillance measures keep the risk of this widespread outbreak at bay.
The experts point out, however, that such stable situation in India requires continued surveillance. "Countries like Pakistan and parts of Africa remain at a high risk because vaccination rates in those areas are much lower," Dr. Siddharth, public health expert, said. Vaccination is an indispensable act in order to avoid the spread of this incapacitating disease that manifests most importantly as a nervous system affliction leading to the paralysis of a long period.
With concerted efforts from health authorities, there is hope someday that the scourge of polio will be completely eradicated from the face of the earth and future generations will never suffer from its effects.
Tamil Nadu Increases Preventative Chickenpox Measures As Cases Rise
Credit: Canva
Tamil Nadu health authorities have ramped up surveillance efforts and implemented preventive and control measures to curb rising chickenpox cases in the state.
The Directorate of Public Health (DPH) and Preventive Medicine has instructed all health officers to intensify active and passive surveillance in all government and private health facilities, schools, colleges and hostels.
They were also directed to ensure that all suspected and confirmed cases are entered into the Integrated Disease Surveillance Programme (IDSP) line list with complete demographic and clinical details.
All medical officers across the state have further been asked to be alert to differentiate chickenpox from other rash illnesses such as measles, rubella, and hand, foot and mouth disease.
This comes weeks after doctors across Pune also warned against the exponential rise in chickenpox cases this winter.
Dr Shirish Kankariya, head of paediatrics at Apollo Hospital Swargate, said he had seen around 15 chickenpox patients in Jan alone, and the current season has brought a visible uptick in cases.
"Chickenpox cases have risen. We are also seeing infections in older children and adult family members, who never had the disease earlier," he told Times of India.
Dr Prateek Kataria, consultant pediatrician and neonatologist at Sahyadri Hospital also noted that out-patient departments (OPDs) have recently seen a large increase confirmed chickenpox cases this year.
He also told the publication: "We are seeing many children with chickenpox in the OPD even among those who have taken both doses of the vaccine. This is expected because the vaccine does not guarantee 100% protection, but vaccinated children usually develop milder illness and do not need hospitalization."
What Is Chickenpox And How Does It Spread?
Chickenpox, caused by the varicella-zoster virus, is extremely contagious and spreads through respiratory droplets or direct contact with someone who is infected. In children, it often starts as a mild rash accompanied by fever, but it can spread quickly in crowded areas.
The virus can also be transmitted through coughing or sneezing, and it is most infectious a day or two before the rash appears and in the early days of the rash. In individuals with weak immunity, the dormant virus may reactivate later in life, causing shingles (herpes zoster).
How To Detect Chickenpox Symptoms Early?
Spotting chickenpox early means looking for general warning signs like fever, fatigue, headache, and loss of appetite, which usually appear one to two days before the rash.
The rash itself starts as tiny red spots that later form fluid-filled blisters and eventually scab over. Paying attention to these early symptoms, especially after known exposure, can help identify the infection sooner.
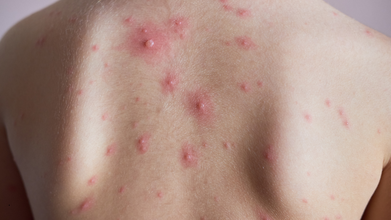
How To Spot A Chickenpox Rash
The first rash usually shows up as small red bumps on the face, chest, or back. These bumps quickly turn into blisters filled with fluid, which are contagious. Over a few days, the blisters break and crust over, forming scabs. It’s common to see spots, blisters, and scabs all at the same time, according to the CDC.
Stages Of Chickenpox
The Mayo Clinic explains that chickenpox progresses in three main stages:
- Incubation Stage (10–21 days): After exposure, the virus remains inactive. Infected individuals typically show no symptoms during this period.
- Prodromal Stage (1–2 days): Early signs include fever, tiredness, headache, loss of appetite, and body aches. This is also when the virus can start spreading to others.
- Rash Stage (5–10 days): Red, itchy spots appear first on the face and chest, spreading across the body. These spots develop into fluid-filled blisters that scab over within a few days. Mild fever, itching, and discomfort are common during this stage.
Breast Cancer To Reach Over 3.5 Mn By 2050, Deaths To Surge 44% Predicts Lancet Study

Credit: Canva
A new study published in The Lancet Oncology journal today revealed that breast cancer continues to be the most common cancer among women worldwide, and predicted that the number of new cases of the deadly disease will reach more than 3.5 million globally in 2050 -- rising by a third from 2.3 million in 2023.
The Global Burden of Disease analysis with data from 204 countries revealed that despite advancements in breast cancer treatments, yearly deaths from the disease will rise by 44 percent -- from around 764,000 to 1.4 million.
While breast cancer disproportionately impacts countries with limited resources, maintaining a healthy lifestyle, including not smoking, getting sufficient physical activity, lowering red meat consumption, and having a healthy weight were found to prevent over a quarter of healthy years lost to illness and premature death.
“Breast cancer continues to take a profound toll on women’s lives and communities,” said lead author Kayleigh Bhangdia from the Institute for Health Metrics and Evaluation (IHME), University of Washington, US.
“While those in high-income countries typically benefit from screening and more timely diagnosis and comprehensive treatment strategies, the mounting burden of breast cancer is shifting to low- and lower middle-income countries where individuals often face later-stage diagnosis, more limited access to quality care, and higher death rates that are threatening to eclipse progress in women’s health,” Bhangdia added.
Inequalities In Breast Cancer Burden
The study revealed that the rates of new cases remain highest in high-income countries (HICs), but are growing fastest in low-income countries (LICs).
Women in low- and lower-middle-income countries accounted for 27 percent (around 628,000) of new cases globally, exposing likely disparities in timely diagnosis and shortages of quality treatment, including radiotherapy machines, chemotherapy drugs, and pathology labs, and standard treatments.
In 2023, an estimated 2.3 million new breast cancers were diagnosed worldwide in women (with 73 percent or 1.67 million cases occurring in high- and upper-middle-income countries). Of these, 764,000 ended in deaths (with 39 percent or 300,000 deaths occurring in low- and lower-middle-income countries).
Further, the number of years of healthy life lost due to poor health and early death more than doubled from 11.7 million years in 1990 to 24 million years in 2023.
Women in low- and lower-middle-income countries also contribute to more than 45 percent of all the ill-health and early deaths from breast cancer globally (nearly 11 million years of healthy life lost).
Three-fold Rise In Pre-menopausal Breast Cancer
The study reported a three-fold rise in pre-menopausal breast cancer in women aged 55 or older in 2023 -- compared to women aged 20-54 years.
However, rates of new cases have risen in women aged 20-54 years (up 29 percent) since 1990, with rates in older women remaining relatively unchanged.
Major Lifestyle Risk Factors
In 2023, 28 percent of the global breast cancer burden (6.8 million years of healthy life lost to disability, illness, and early death) was linked to six potentially modifiable risk factors. These include:
- High red meat consumption -- linked to nearly 11 percent of all healthy life lost
- tobacco use (including second-hand smoke; 8 percent),
- high blood sugar (6 percent),
- high body mass index (4 percent),
- high alcohol use and low physical activity (both 2 percent)
Substantial progress has been made in reducing the global breast cancer burden linked to high alcohol use and tobacco between 1990 and 2023, which declined by 47 percent and 28 percent, respectively.
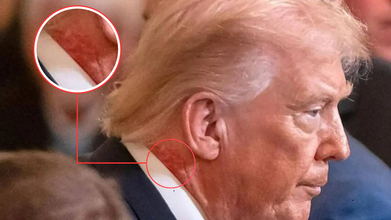
Donald Trump's Neck Appears To Have Redness And Rashes, White House Says A Skin Cream Caused It
Credits: AP
Donald Trump is again in the news, this time not for his cognitive conditions and speculations around it, but for a skin rash and redness around his neck. During a Medal of Honor ceremony in the East Room of the White House, many pointed out the redness around his neck and asked if there was any underlying medical reason to it. The close-up photos shows clear red marks around the 79-year-old president's neck.
Also Read: Breast Cancer To Reach Over 3.5 Mn By 2050, Deaths To Surge 44% Predicts Lancet Study
Why Does President Trump Have Red Rashes Around His Neck?
As per the White House, this redness around his neck is due to a skin cream he has been using. However, the White House has not given any specific reason behind the treatment for which the skin cream has been prescribed. "President Trump is using a very common cream on the right side of his neck, which is a preventative skin treatment, prescribed by the White House Doctor,” Sean Barbabella, the president's doctor, said in a statement. “The President is using this treatment for one week, and the redness is expected to last for a few weeks.”
No follow-up questions on the treatment were given, neither any clarity was given on the condition. However, zoomed in photos from as far back as the Board of Peace meeting at the US Institute of Peace on February 19 too show the redness on President's neck.
Topical treatments can sometimes lead to temporary skin irritation, flushing, or redness - especially if they contain active ingredients like retinoids, benzoyl peroxide, alpha hydroxy acids, or prescription-strength dermatological compounds.
Trump’s medical report from his April 2025 physical exams has noted he was on mometasone cream “as needed” for an unspecified skin condition.
Also Read: Ashley Dalton Diagnosed With Incurable Breast Cancer Stands Down From Her Position
Why Do Skin Cream Cause Redness And Rashes?
While this is not true for all creams, some skin cream could make your skin red by increasing the turnover of cells that stimulate collagen production or reduce inflammation.
In this process, the skin may appear red, slightly inflamed, or sensitive – especially in areas like the neck, which tends to have thinner skin than the face.
Common reasons for redness caused by skin cream include:
- Irritant contact dermatitis
- Allergic reaction to ingredients
- Increased blood flow from active compounds
- Over-application of product
- Sun sensitivity after use
- Skin around your neck may especially react more visibly than other areas due to its delicate texture.
When should redness be a concern?
Even though temporary redness from skincare products is usually harmless, doctors say you must always use creams after a prescription and reach out to them in case of:
- Severe burning or itching
- Blistering or peeling
- Swelling of the face or throat
- Symptoms lasting more than a few days
- Signs of infection
- In most cases, discontinuing the product allows the skin to return to normal within a short period.
© 2024 Bennett, Coleman & Company Limited

