- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
More High School Students Are Skipping Their Breakfast, Finds Study

Credits: Canva
Is your teenager skipping breakfast? Why is that happening and what can you do? As per the data from the Centers for Disease Control and Prevention (CDC), which surveyed adolescent health and well-being found that 1 in 4 students in high school ate breakfast, which means 3 in 4 high school students are not eating their breakfast. This data is as per the 2023 survey.
What Did The Study Intend?
The report describes a 10-year long trend and also recent changes among the two years. The study delved deeper into adolescents' dietary, physical activity and sleep behaviors. The study is also based on a national youth risk behavior survey of a representative sample of students from grade nine to 12.
What Did The Study Find?
The study found that while high school students drank slightly less soda and sports drinks and consumed more water, other healthy eating habits declined. In 2023, only 27% of students ate breakfast every day in the past week. The numbers were even lower for female students, with just 22% eating breakfast daily, compared to 32% of male students. Boys were also more likely to eat fruits and vegetables daily and drink water at least three times a day. Poor mental health and lack of physical activity have also been linked to skipping breakfast.
The other findings included a survey across 10-year period, where a decrease in the percentage of students eating fruits from 65% to 55%, eating vegetables, from 61% to 58%, and having breakfast daily from 38% to 27% was noted.
However, there was a positive trend among this, which was in children drinking plain water at least three times a day, which increased from 49% to 54% from when the survey began in 2015.. There were fewer students who also said that they drank soda in 2023 than in 2013. On an average, in 2013, around 22% students avoided soda, whereas in 2023, 31% students avoided it.
The report also emphasized that a healthy diet, along with daily physical activity and sufficient sleep further contributes to a healthy lifestyle. “The 10-year trends from 2013 to 2023 also show a decline in healthy dietary, physical activity, and sleep behaviors,” the survey reported.
Why Do High School Students Skip Breakfast?
While there is no one straightforward answer to it, psychologists and those who study children, believe that for many high school going kids, it is the easiest time to skip a meal. This is because they are caught between rushing to school, or not just that hungry in the morning. So for them, to sit down to have a breakfast may seem hassle and something they would have to take time out from their busy schedule. They at this age also prioritize their extra-curricular activities.
There has also been a shift in their circadian rhythm, and most teens cannot fall asleep before 11 pm, or even at midnight. Which means they wake up tired and struggle to do things right in the morning, which is why they choose to skip breakfast or give extra minutes to any other activities.
There is of course another, more popular reason, to lose weight. While experts and studies, like the one published in the Journal of Nutrition that found skipping breakfast leads to higher levels of hunger hormones, the students still feel the need to do this. However, it could lead to a slow metabolism, prompt the body to conserve energy and burn fewer calories, weight gain and deprive yo off the essential nutrients like calcium, iron, and vitamin D.
Without a morning breakfast, your blood sugar might drop too, which can increase irritability and stress, along with including the risk of depression in teenage.
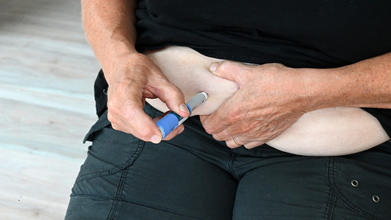
Novo Nordisk And Hims & Hers Health End Dispute, Plan to Sell Wegovy Together
Credit: iStock
Wegovy maker Novo Nordisk has ended its legal dispute with the Hims & Hers Health platform, according to a media report.
The two companies are likely to partner and sell the blockbuster obesity drug Wegovy together on the Hims & Hers Health platform, Bloomberg News reported.
Market analysts have expressed surprise on the move, as both Novo and Hims have been previously engaged in legal battle over Wegovy.
“There is no other way to describe the Hims news as both a surprise and an unabashed positive for Hims' stock,” Leerink Partners analyst Michael Cherny was quoted as saying in a note to clients.
A Novo spokesperson said in an emailed statement that the company is "always in conversation with companies that can help improve patient access to FDA-approved medicines".
The Dispute Between Novo and Hims
In February, Novo sued Hims for launching a similar version of its new Wegovy weight-loss pill for $49.
The Danish drugmaker accused Hims of patent infringement on Semaglutide -- the active ingredient behind its best-selling medications Ozempic and Wegovy.
Semaglutide is popularly known for weight loss , but is also effective for diabetes and is used primarily for that.
The US Food and Drug Administration (FDA) had also threatened action against Hims.
Last year, Novo had to end a short-lived agreement to sell Wegovy over Him's marketing tactics and continued sales of copycat versions of Wegovy.
Recently, the FDA has signaled plans to crack down on the proliferation of copycat, or compounded, weight-loss drugs.
Wegovy And Ozempic To Cost Less In 2027
Currently, Wegovy injections and pills cost $1,349.02 a month, whereas Ozempic and Rybelus cost $1,027.51, Novo told PEOPLE.
Individuals with commercial insurance pay $25 a month, whereas those using cash pay between $149 to $499. Patients on Medicare will pay $274 per month.
Late in February, Novo Nordisk announced it would slash the price for all doses:
- The list price of Wegovy injections and the new Wegovy pill will be cut in half
- Ozempic injections will be cut by 35 percent
- The semaglutide tablet Rybelsus will now cost $675 a month
"There are more than 100 million people living with obesity and over 35 million with type 2 diabetes and, and for some, list price has been a real barrier to access and affordability," Jamey Millar, Executive Vice President, US Operations of Novo Nordisk Inc., was quoted as saying to PEOPLE.
Indian Drugmakers Rush For Generic Weight Loss Drugs
Meanwhile, amid the patent expiration of semaglutide, several pharma companies in India are planning big launches of Wegovy's generic versions.
Several leading drugmakers have already secured regulatory approval or recommendations to produce and market generic versions of the weight loss drugs in the country.
Hyderabad-based Dr. Reddy's already applied for a trademark with the brand name Obeda and a logo.
Other companies like Sun Pharma, Zydus Lifesciences, and Nacto Pharma are also entering the rat race of launching multiple generic versions to make the treatment more affordable for patients with obesity and weight-related health risks.
Sun Pharma also announced the plans for "day-one" launches of generic prefilled pens.
US FDA Vaccines Chief Vinay Prasad Exits Again After Criticism Over Drug Application Handling

Credit: USFDA
The US Food and Drug Administration (FDA)’s Vinay Prasad has once again — for the second time in less than a year — stepped down from his post as director of the agency’s Center for Biologics Evaluation and Research, amid controversies over the review of vaccines and specialty drugs for rare diseases.
Announcing the news to FDA staff in an email late Friday, FDA Commissioner Marty Makary said Prasad would depart at the end of April. Makary added that Prasad would return to his academic position at the University of California, San Francisco (UCSF).
Taking to social media platform X, Makary said that under Prasad’s leadership, his center recorded a record number of approvals in December.
“A year ago, Dr. Prasad came to the FDA to implement four major long-lasting reforms: a 2-to-1 pivotal trial requirement, national priority reviews, a risk-stratified COVID vaccine framework, and the new plausible mechanism framework for ultra-rare diseases, which we launched last week,” Makary said.
The FDA commissioner noted that Prasad “got a tremendous amount accomplished within his one-year sabbatical from UCSF and will be returning to his academic home later next month,” and thanked him “for his service and personal sacrifice in taking time away from his family.”
The FDA is expected to announce Prasad’s successor before his departure.
Who Is Vinay Prasad?
Vinay Prasad is an Indian-origin American hematologist-oncologist and author. He was first appointed as the FDA’s vaccines chief in May 2025.
Prasad, known as a longtime critic of the FDA’s standards for drug reviews, drew controversy for raising the bar for new drug approvals. The move did not sit well with pharmaceutical companies and reportedly dashed the hopes of some patients with rare diseases.
In July, he was removed from his position following disputes with biotechnology executives, patient organisations, and conservative allies of US President Donald Trump. He was later reinstated after Makary and US Health Secretary Robert F. Kennedy Jr. pushed for reconsideration.
What Is The Controversy?
While Prasad, along with Makary, announced several measures to make FDA drug reviews faster and easier for companies, he also imposed new warnings and study requirements for some biotech drugs and vaccines.
This was particularly evident in the case of COVID-19 vaccines, which have been a target of criticism from Kennedy, who was a longtime anti-vaccine activist before joining the Trump administration.
The latest controversy involves the FDA’s interactions with Dutch biopharma company uniQure, which developed an experimental gene therapy for Huntington’s disease that is injected directly into the brain during a surgical procedure.
Huntington’s is a deadly neurological condition affecting about 40,000 Americans, and currently has no cure.
UniQure faced a setback after Prasad’s centre said its earlier studies were insufficient to support a biologics licence application.
During an earnings call earlier this week, the company said the FDA was demanding a new trial involving sham surgery for some patients.
Executives said the request for a sham-controlled trial contradicted earlier FDA guidance. They also questioned whether such an approach would be ethical for patients with Huntington’s disease, which is progressive and ultimately fatal, typically in middle age.
Earlier, Prasad also refused to allow the FDA to review a highly anticipated flu vaccine from Moderna made using mRNA technology.
The rejection of the application -- highly unusual for the FDA -- prompted Moderna to go public with Prasad’s decision and vow to formally challenge it.
A week after the rejection became public, the FDA reversed course and said it would accept the vaccine for review, pending an additional study from the company.
Prasad’s handling of rare-disease therapy applications also drew criticism after the FDA asked Sarepta Therapeutics, a drugmaker developing treatments for Duchenne muscular dystrophy, to pause shipments following reports of patient deaths.
The company initially resisted, wanting to continue distributing treatments for patients who could still walk, but later agreed. The agency, however, reversed the pause just days later.
Duchenne muscular dystrophy affects a small number of boys and young men who typically lose their ability to walk before puberty and often die by around age 30.
Health Minister JP Nadda Announces Cervical Cancer Screenings At Ayushman Arogya Mandirs

Credit: PIB
Union Health Minister JP Nadda has announced that cervical cancer screenings using Visual Inspection with Acetic Acid (VIA) will now be available at Ayushman Arogya Mandirs and other health facilities for women between 30 and 65 years of age.
"Screening for cervical cancer is now available at 1,81,000 Ayushman Arogya Mandirs, also known as Health and Wellness Centers, across the country as a part of population based screening for early detection and treatment," said Nadda, while addressing a press briefing at the World Health Organization virtually.
Using VIA, a low-cost, point-of-care method, trained health workers will screen women for cervical cancer. Those who test positive will then be referred to higher centers for diagnostic confirmation and further evaluation.
Nadda also shared that the cervical cancer screening in the country has been expanded as part of comprehensive primary healthcare under the National Program for Prevention and Control of Non-Communicable Diseases (NP-NCD).
"Over 86 million women have already been screened for cervical cancer under the program, reflecting India’s sustained commitment to early detection and prevention," Nadda informed.
Despite being highly preventable as well as treatable, cervical cancer is a public health concern in India.
The country loses one women every eight minutes to cervical cancer.
As per the World Health Organization (WHO) Director-General Tedros Adhanom Ghebreyesus, about 42,000 new cases of cervical cancer is reported annually in India. This underscores the need for preventive measures such as vaccination and early screening.
The WHO Global Strategy to eliminate cervical cancer includes the 90-70-90 targets by 2030 -- vaccinating 90 per cent of girls against HPV, screening 70 percent of women, and ensuring treatment for 90 percent of those diagnosed with cervical disease.
In line with the global strategy to fight cervical cancer, Prime Minister Narendra Modi recently also launched a free HPV vaccination drive that will target health and well being of adolescent girls in the country.
What Is Cervical Cancer?
Cervical cancer develops in a women's cervix (uterus opening) due to abnormal cell growth, primarily caused by persistent HPV infection, a common infection that's passed through sexual contact.
When exposed to HPV, the body's immune system typically prevents the virus from causing damage however, in a small percentage of people, the virus can survive for years and pave the way for some cervical cells to become cancerous.
Treatment involves surgery, radiation, and chemotherapy, with early detection significantly improving outcomes, though it remains a major cancer in low-income countries Cervical cancer can also be prevented through vaccination and regular screening (Pap/HPV tests).
Symptoms Of Cervical Cancer
Cervical cancer has no symptoms in the early days and therefore, is hard to detect until it has spread. However, the early-stage symptoms include:
- Vaginal bleeding after sex
- Vaginal bleeding post-menopause
- Vaginal bleeding between periods or unusually heavy/long periods
- Watery vaginal discharge with a strong odour or containing blood
- Pelvic pain or pain during intercourse
- Advanced Cervical Cancer Symptoms (when cancer has spread beyond the cervix)
- Painful or difficult bowel movements or rectal bleeding
- Painful or difficult urination or blood in the urine
- Persistent dull backache
- Swelling of the legs
- Pain in the pelvis or lower abdomen
How Can Cervical Cancer Be Prevented?
Cervical cancer is largely preventable and, when detected early, it is highly treatable. The WHO recommends HPV vaccination for girls aged 9 to 14, before they become sexually active, along with regular cervical screening from age 30, or 25 for women living with HIV.
Despite this, unequal access to vaccination, screening and treatment continues to drive higher rates of illness and deaths in regions such as sub-Saharan Africa, Central America and Southeast Asia.
© 2024 Bennett, Coleman & Company Limited

