
Image Credit: CDC
Lyme Disease’s ‘Achilles’ Heel’ Discovered- Could The Bacteria Itself Be Used For Treatment?
Lyme disease has been a challenge for researchers and doctors for decades. Despite of antibiotic therapies, many patients continue to suffer from debilitating complaints like fatigue, arthralgias, and cognitive impairment—posing stern questions about more efficacious treatment. Researchers may now have a vital piece of the puzzle.
For years, scientists have been working to find a definitive cure, with existing treatments coming up short in chronic cases. But here's the thing: scientists have recently discovered a potential weak spot in Borrelia burgdorferi, the bacterium that causes Lyme disease.
A recent study published in mBio, published by the American Society for Microbiology, has uncovered a key enzyme—lactate dehydrogenase (BbLDH)—that the bacterium cannot live without. The finding opens the door to new, extremely targeted therapies that might revolutionize the treatment of Lyme disease forever.
Why is Lyme Disease a Growing Public Health Concern?
Lyme disease is not some other tick bite; it is the most prevalent tick-borne disease in America and Europe and infects thousands of individuals each year. Increases in tick infestations by climate change and urbanization only worsen the issue.
Although early Lyme disease can be cured with antibiotics, most patients have residual symptoms, commonly called post-treatment Lyme disease syndrome (PTLDS). With antibiotic resistance looming as a large problem, scientists have been looking for other ways to treat the disease—and this new finding could be the answer they've been seeking.
What Is the Role of Lyme Disease Enzyme?
Bacteria mostly use thiamin, a survival cofactor, but B. burgdorferi does not. It instead uses BbLDH to reduce pyruvate to lactate, a process necessary for balancing its energy supply. This is a metabolic idiosyncrasy specific to B. burgdorferi and provides a good drug target.
A group of researchers at Virginia Commonwealth University employed genetic, biochemical, and structural analyses, such as X-ray crystallography, to establish that BbLDH is essential for bacterial viability. Their findings demonstrated that in the absence of this enzyme, B. burgdorferi is unable to grow or infect hosts, which offers a very promising new avenue for treatment.
Understanding BbLDH’s importance, researchers launched a high-throughput drug screening process to find compounds that could inhibit its function. Among the tested substances, four promising inhibitors were identified—two of which successfully halted B. burgdorferi growth without harming human cells. These findings mark a major step toward creating a targeted Lyme disease treatment, reducing dependence on broad-spectrum antibiotics.
Why This Discovery Matters for the Lyme Disease Treatment?
Targeting BbLDH directly weakens B. burgdorferi, which may allow for more effective Lyme disease treatments, and this finding a treatment breakthrough. By targeting this particular enzyme, scientists can create specific medicines that could enhance patient results. Moreover, with fewer broad-spectrum antibiotics required, antibiotic resistance and adverse side effects might be reduced, providing a safer and more streamlined treatment.
In addition to direct therapeutic progress, knowledge of BbLDH's function could also assist in the creation of a potential Lyme disease vaccine, offering long-term immunity to the infection. Additionally, the results could find applications beyond Lyme disease, facilitating research into corresponding metabolic weaknesses in other tick-borne diseases, leading to potential wider applications in infectious disease treatment.
With thousands of individuals afflicted by chronic Lyme disease symptoms, this advance brings new hope. Next steps will include optimizing these BbLDH inhibitors, further laboratory research, and ultimately moving to human clinical trials. With success, these precision therapies have the potential to transform Lyme disease treatment, paving the way for a brighter future for them.
This finding isn't simply the next scientific breakthrough—it's a possible lifeline for Lyme disease sufferers around the globe. By identifying BbLDH as a vital weak link in B. burgdorferi, scientists are closer than ever before to creating an exact, effective cure. Although there is still more to be done, this discovery marks a new era in the battle against Lyme disease, moving the medical community one step further toward a much-desired cure.
What is Lyme Disease?
Lyme disease is a bacterial infection by Borrelia, spread to humans by an infected tick's bite. People who spend much time in grassy, brushy, or wooded areas are more likely to develop the disease. But precautions taken in such places can decrease the chances of infection considerably.
Symptoms of Lyme Disease
A tick bite will usually look like a small, itchy bump on the skin, just like a mosquito bite. This does not, however, confirm Lyme disease, and some people might not even notice they have been bitten. The symptoms of Lyme disease evolve in stages, although they may overlap, and some people may not notice the early signs that are typical.
Stages of Lyme Disease
Stage 1 (Early Localized Disease)- One of the most prevalent early manifestations of Lyme disease is a rash, although it is not seen in every case.
Stage 2 (Early Disseminated Disease)- Untreated Lyme disease will progress 3 to 10 weeks following the initial tick bite. During this stage, the symptoms worsen and become more widespread, targeting various parts of the body.
Stage 3 (Late Disseminated Disease)- During the advanced stage, patients will have chronic symptoms from previous stages, and other complications will occur. In the most serious cases, Lyme disease can cause damage to tissue or joints, necessitating intensive medical treatment.

Credits: Canva
Trump Administration Cuts LGBTQ Health Research Grants: What It Means
Last week, the government of the United States abruptly terminated at least 68 grants which were awarded to 46 institutions. This was a total of $40 million in funding. These grants were supporting research which was related to LGBTQ health, and included HIV prevention, youth suicide, cancer and bone health. While some of these funds have already been used, at least $1.36 million was withdrawn from future support. This figure is also an undercount, as estimates were available only for a portion of the cancelled grants.
This has affected a large number of studies, especially on health of sexual minorities that has led to many researchers to believe that the cuts are also politically motivated. The National Institute of Health (NIH), which had earlier awarded these grants falls under the Department of Health and Human Services (HHS). The HHS spokesperson Andrew Nixon defended the move stating that the agency aims to uphold "gold-standard, evidence-based science". Now this does not necessarily comes as a shock to people, as every since the Trump administration has taken over, many studies, grants, and papers have been removed, erased and or cancelled wherever there was gender involved.
Earlier this month, two California researchers have alleged that the US Government health journal instructed them to remove references to gender and sexual orientation from a scientific manuscript that had already been accepted for publication. Their paper examine smoking habits among the youth in the rural areas and was set to be published in Public Health Reports, the official journal of the US Surgeon General and the US Public Health Service.
The Impact On LGBTQ Health Research
One of the most affected studies was a long-term project at the Vanderbilt University which tracked the health of more than 1,200 LGBTQ individuals aged 50 and older. This was led by Tara McKay of Vanderbilt's LGBTQ+ Policy Lab. The research had already yielded two dozens of published papers and has helped improve healthcare for LGBTQ patients by training doctors in inclusive medical practices. The study's funding was up for its renewal in April, however, it is now in jeopardy.
Another researcher from the University of Minnesota saw his research on cancer in gay and bisexual men come to an abrupt halt. The cuts may have long-term consequences, and may slow scientific discovery and eliminate jobs for young researchers who want to enter this field.
Trump Administration's Stance On Gender In Healthcare
These cuts are not an isolated event but part of a broader pattern of policy changes under the Trump administration that have targeted LGBTQ rights in healthcare. Trump’s executive orders and administrative actions altered the legal definition of gender in federal health policies, rolling back protections for transgender and non-binary individuals.
One major change came in 2020 when the administration reversed an Obama-era rule under the Affordable Care Act that prohibited healthcare discrimination based on gender identity. This move allowed medical providers to refuse treatment to transgender individuals. Additionally, HHS redefined "sex" in a way that excluded protections for gender identity, making it harder for transgender people to access gender-affirming care.
The Justification
Termination letters too have been sent to researchers citing that their research is either "unscientific" or do not contribute to health of "many Americans".
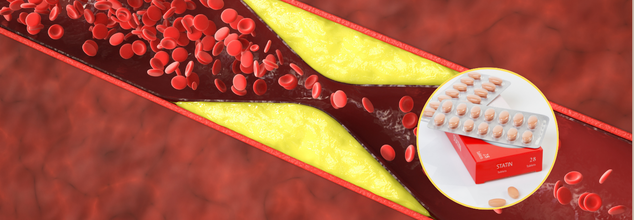
Cholesterol- Lowering And Statin Drug Combo Could Cut Fatalities By 49%, Saving Thousands Of Lives, Study Finds
Cardiovascular diseases remain the number one cause of death across the world, claiming an estimated 18 million lives every year. Although statins have been the first line of treatment for reducing cholesterol levels for years. They achieve this by suppressing an enzyme in the liver that is used to make cholesterol, thus lowering low-density lipoprotein cholesterol (LDL-C), or 'bad' cholesterol. Statins are not enough for some patients, though, and this is where ezetimibe steps in.
According to a study in the Mayo Clinic Proceedings, a combination of two cholesterol-lowering medications—statins and ezetimibe—may save thousands of lives every year by cutting heart attacks, strokes, and other cardiovascular events by half. This study contradicts the traditional practice of prescribing statins alone and recommends the use of combination therapy immediately in high-risk patients.
Ezetimibe has a different mode of action than statins as it inhibits the absorption of cholesterol in the gut, complementing the effect of the statins. The new research uncovers that prescribing both drugs concurrently from the start, instead of adding ezetimibe subsequently, yields considerably superior results for high-risk patients with cardiovascular disease.
The largest study ever conducted, it pooled data from 14 earlier trials involving 108,353 patients. These patients were at 'very high' risk of developing a heart attack or stroke, or had already developed one. The results strongly support the need to modify treatment strategies in high-risk patients.
The study discovered that the patients who underwent the combination therapy had a 19% lower risk of dying from any cause. There was also a 16% decline in cardiovascular death, which pointed to the substantial effect of the therapy on the heart. The study further found that the major adverse cardiovascular events, such as heart attacks and strokes, decreased by 18% and 17%, respectively. These findings reinforce the effectiveness of combining cholesterol-lowering drugs in reducing mortality and preventing life-threatening cardiovascular complications.
LDL-C levels dropped significantly more in those receiving the combination treatment, increasing the likelihood of reaching the target level of under 70mg/dL by 85%.
Traditionally, statins alone have been prescribed for years, and the physician would later evaluate if extra medicine is required. But based on the findings of the study, physicians ought to prescribe the two drugs at once to those patients who are high-risk patients. This change can save more than 330,000 deaths a year across the globe, including almost 50,000 fatalities in the United States alone, says the study's lead author, Prof. Maciej Banach from Poland's John Paul II Catholic University of Lublin.
This study is conclusive that combination cholesterol-lowering therapy must be entertained forthwith and must be the treatment of choice for very high-risk patients following an acute cardiovascular event," said Prof. Peter Toth, one of the authors of the study.
The strategy not only has the potential to save lives but may also lead to substantial cost savings for healthcare systems through decreased hospitalization and complications from untreated cardiovascular disease. Prevention of heart failure, recurrent strokes, and other catastrophic events would reduce the economic burden of long-term cardiac care.
How Does The Drug Combination Affect Treatment for Cardiovascular Diseases?
High levels of LDL-C contributed to 4.5 million deaths in 2020 independently, with most deaths occurring in Eastern Europe and Central Asia. With the prevalent nature of cardiovascular disease worldwide, implementing combination therapy as the mode of treatment might have far-reaching advantages.
Since statins and ezetimibe are already on the market in clinical practice, implementation of these findings should be relatively easy. Scientists hope that this evidence will be followed by a revision of medical guidelines, motivating healthcare professionals across the globe to become more proactive.
The authors of the study emphasize the need for changing current treatment regimes. Doctors are urged to begin patients on combination therapy immediately, and not to wait and observe whether statins alone can work. Immediate and forceful treatment might be the difference between life and death for thousands of people every year.
"Our results emphasize the wisdom of the saying 'the lower the better for longer,' but also the not-less-important 'the earlier the better' in the treatment of high-risk cardiovascular patients," Toth said.
For individuals, this news is a wake-up call to talk with their healthcare provider about cholesterol control strategies. Knowing the value of combination therapy and fighting for full treatment options could have a major impact on long-term health.
The new evidence is a strong argument for the routine application of combination therapy in controlling cholesterol levels for patients at risk. With a solid body of evidence in favor of its application, physicians everywhere are encouraged to implement this treatment to cut the death toll from cardiovascular disease. As guidelines continue to change, the universal use of this practice could be a new dawn for the prevention of heart disease, saving half a million lives each year.
Credits: Canva
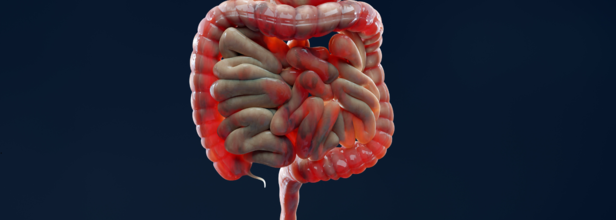
FDA Approves Tremfya For Active Crohn's Disease
Of the many things new, the US Food and Drug Administration (FDA) has approved Tremfya (guselkumab) for adult patients with moderately to severely active Crohn's disease.
What is Crohn's Disease?
Crohn's disease is a type of inflammatory bowel disease (IBD) that causes swelling and irritation of the tissues, called inflammation, in the digestive tract. This can lead to belly pain, severe diarrhea, fatigue, weight loss and malnutrition.
What does Tremfya do?
It contains the active ingredient guselkumab, which is a prescription drug to treat moderate to severe plaque psoriasis, psoriatic arthritis, and ulcerative colitis in adults, recently it has been approved for Crohn's disease.
Guselkumab targets the IL-23 subunit alpha which prevents it from binding to cell receptors that would otherwise be activated by its presence. It was first developed by Janseen Pharmaceuticals and in November 2016, a Biologics License Application to FDA was submitted to seek for its approval.
Tremfya blocks interleukin-23 or IL-23, which is a cytokine responsible for inflammation and binds to CD64, which is a receptor on cell that produce IL-23. The CD64 cells are the source of IL-23, which causes inflammation in Crohn's disease.
This approval is the fourth indication for this dual IL-23 inhibitor in the United States, which makes Tremfya the first and only IL-23 inhibitor that offers subcutaneous and intravenous induction options for adults with Crohn's disease.
How Was It Approved?
The approval is based on the data from a 3-phase trial. In the GRAVITI study, 400mg of Tremfya was administered through subcutaneous induction at 0,4,and 8 weeks. This led to a significantly higher clinical remission and endoscopic response when compared with the placebo. The clinical remission was 56% while placebo noted for only 22%, whereas endoscopic response was 35% and placebo had a 15% impact. Similar results were also seen in the second and third phase of trials which compared Tremfya 200mg intravenous induction at weeks 0,4, and 8 with placebo.
"Despite the progress in the management of Crohn's disease, many patients experience debilitating symptoms and are in need of new treatment options," lead investigator of the GRAVITI study, Remo Panaccione, M.D., from the University of Calgary in Alberta, Canada, said in a statement. "Importantly, the fully subcutaneous regimen offers choice and flexibility for patients and providers that have not been available before."
What Are The Symptoms Of Crohn's Disease?
- Diarrhea
- Fever
- Fatigue
- Belly pain and cramping
- Blood in the stool
- Mouth sores
- Reduce appetite and weight loss
- Pain or damage near or around the anus due to inflammation
It can also affect any part of the small or large intestine and could in fact involve multiple segments or may be continuous. It most commonly involves the last part of small intestine. In some people, the disease is only in the colon or the large intestine.
While symptoms of Crohn's disease can range from mild to severe, they usually develop gradually. However, sometimes it may also come suddenly without a warning. So, someone with Crohn's disease may have periods of time without any symptoms, called remission.
© 2024 Bennett, Coleman & Company Limited

