- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories

FDA Approves ZYN Nicotine Pouches As Safer Alternative For Adult Tobacco Users
The U.S. Food and Drug Administration (FDA) has taken a significant step in the evolving landscape of tobacco harm reduction by authorizing the marketing of 20 ZYN nicotine pouch products. The agency released this decision following comprehensive scientific review under the PMTA pathway, marking the first time FDA has authorized nicotine pouches. This move, while bringing to light debates concerning public health, could fundamentally shape the future of nicotine consumption and alternatives to tobacco use in the United States.
ZYN nicotine pouches are innovative products designed as alternatives to traditional tobacco. Unlike cigarettes or smokeless tobacco, these small synthetic fiber pouches contain nicotine but no tobacco. Users place them between the gum and lip, where nicotine is absorbed into the bloodstream. Available in various flavors, including cinnamon, citrus, and cool mint, ZYN offers two nicotine strengths- 3 milligrams and 6 milligrams.
This new product category targets adult smokers who would like to switch to less harmful alternatives compared to traditional tobacco. FDA's authorization marks a landmark moment for this emerging segment of the tobacco market in the United States.
Why Did the FDA Approve ZYN?
The FDA decision anchors from the Family Smoking Prevention and Tobacco Control Act of 2009, which obligates all new tobacco products to comply with strong public health standards. The agency review found that the ZYN pouches involve substantially lower risks compared to cigarettes and smokeless tobacco products, such as snuff or snus.
The pouches contain far fewer harmful constituents associated with cancer and other life-threatening diseases. In addition, studies submitted by the manufacturer showed that many adult smokers and users of smokeless tobacco switched entirely to ZYN, indicating its potential as a harm-reduction tool.
"These nicotine pouch products meet that bar by benefiting adults who use cigarettes or smokeless tobacco products," said Matthew Farrelly, Ph.D., director of the FDA's Office of Science at the Center for Tobacco Products.
How This Will Help Manage Youth Tobacco Use?
This is a clear FDA warning: ZYN is not risk-free, nor is it "FDA-approved." Although the agency said that low use levels of nicotine pouches among youths remain at 1.8% of middle and high school students in the United States who reported using any tobacco product, according to the 2024 National Youth Tobacco Survey, the agency emphasized preventing youth exposure.
The FDA did this by applying stringent marketing restrictions, including the following:
- Ban mass-market advertising on TV and radio.
- Require actors/models in ads to appear at least 35 years old.
- Avoid themes, characters, or imagery appealing to youth.
The agency will also closely monitor marketing practices and suspend or withdraw authorizations if products no longer meet public health standards.
ZYN pouches don't contain any tar and the carcinogenic chemicals present in cigarette smoke and in the majority of smokeless tobacco products. Unlike snus, a popular form of pasteurized tobacco widely used in Scandinavian countries, ZYN contains no tobacco at all. In fact, that positions it like the nicotine gum or lozenge, releasing controlled doses of nicotine to eliminate craving.
For decades, tobacco companies have been looking for alternatives to traditional cigarettes, as smoking rates in the U.S. and around the world have declined. E-cigarettes became popular in the early 2010s but were later criticized for underage vaping. ZYN nicotine pouches could be a safer alternative, but critics fear they may follow the same path if youth use increases.
ZYN, marketed by Philip Morris International acquisition target Swedish Match through 2022 for a cost of $16 billion. While Philip Morris invests massively into nicotine pouches, huge demand from customers bolsters the business segment in the most phenomenal way in U.S. tobacco. Already nicotine pouches emerge as a pace-setter that could set to be at the core of industry's future prospects.
Also Read: 3 Science-Backed Methods To Quit Smoking For Good
However, controversies run rampant in the history of tobacco alternatives. For example, e-cigarette giant Juul Labs was sued for marketing to minors, settling in 2023 for $462 million without admitting wrongdoing. Critics are now beginning to scrutinize ZYN flavored products, including citrus and peppermint, arguing they may attract younger users.
That benefit, however – the FDA seems poised to acknowledge adult smokers will use nicotine pouches – underscores much-needed responsible marketing and rigorous oversight. Public health advocates caution, however, there is no such thing as winning when it has to be over youth uptake first and close to long-term effects on health.
Supporters say that ZYN is a genuine substitute for smokers looking to quit or reduce their reliance on devastating tobacco products. Indeed, as Brian King, Ph.D., FDA's Center for Tobacco Products director, noted, "It is imperative that the maker of these products does so responsibly so that youth will not take up their use".
The FDA's approval of ZYN nicotine pouches is part of a larger effort to regulate and innovate within the tobacco industry. Over the past decade, the agency has received applications for nearly 27 million products, authorizing only those that meet rigorous health standards.
This also goes with recent suggestions to limit nicotine content in cigarettes, marking a step toward harm reduction and prioritization of public health. However, challenges remain, but the ZYN authorization is reflective of a growing recognition of alternative nicotine products' role in reducing tobacco-related disease and mortality.
The FDA's approval of ZYN nicotine pouches marks a turning point in tobacco harm reduction. As a less harmful alternative to cigarettes and smokeless tobacco, ZYN has the potential to benefit adult smokers while reducing risks to public health. However, the success of this initiative will depend on strict regulatory oversight, responsible marketing, and continued vigilance against youth use.
Effective Tips for Quitting Smoking
Smoking cessation is one of the best heart-healthy lifestyle choices you could ever make and minimize the risk for developing conditions like A-Fib. While quitting smoking might not be an easy task, its benefits can be felt at once and significantly. Here is how to quit smoking successfully.
1. Decide on a quit date
Set a quit date and be firm about it. This will give you ample time to mentally and physically prepare for the transition.
2. Build a Support System
Share your decision with your family, friends, or support group. This will make it easier for you because you have people to motivate and hold you accountable.
3. Use Nicotine Replacement Therapy
Consider using nicotine patches, gum, or lozenges to manage withdrawal symptoms and reduce cravings. Seek the advice of a healthcare professional.
4. Identify and Avoid Triggers
Identify situations or emotions that make you want to smoke, such as stress, boredom, or social settings. Develop healthy coping strategies, like journaling or engaging in a hobby, to navigate these triggers.
5. Stay Active
Engage in regular exercise. Exercise not only diverts your mind from excessive hunger and craving but also improves moods and overall well-being.
6. Keep Hydrated
Drinking water will help to flush out nicotine from your system, and the level of your craving will decrease.
7. Stress Management
People often smoke when their stress is too high. Try to engage in some relaxing activities like yoga, meditation, or deep breathing.
Tobacco Product Use Among Middle and High School Students — National Youth Tobacco Survey, United States, 2024
E-Cigarette and Nicotine Pouch Use Among Middle and High School Students — United States, 2024
Credits: Canva
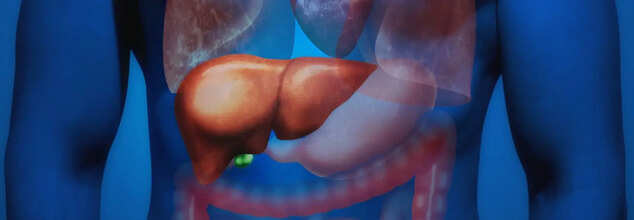
A New Blood Test Predicts Fatty Liver Disease 16 Years Ahead Of Diagnosis
Fatty liver disease, now called metabolic dysfunction-associated steatotic liver disease (MASLD), happens when extra fat builds up in liver cells without alcohol being the cause. Normally, the liver contains some fat, but if more than 5–10% of the liver’s weight is fat, it is considered a fatty liver. In its advanced stage, MASLD can develop into metabolic dysfunction-associated steatohepatitis (MASH), causing swelling and serious damage to the liver. Alarmingly, MASLD affects about 100 million people in the United States, including a growing number of children.
The disease often develops silently. Many people do not experience symptoms early on, but if it worsens, signs like fatigue, abdominal pain, jaundice, swelling, and even mental confusion may occur. Risk factors include obesity, diabetes, high cholesterol, rapid weight loss, poor eating habits, and certain medications.
A Breakthrough in Early Prediction
A new study offers hope for earlier detection. Researchers, led by Dr. Shiyi Yu from Guangdong Provincial People's Hospital in China, have developed a blood test that looks for five specific plasma proteins to predict MASLD years before symptoms show up. The findings are set to be presented at the Digestive Diseases Week meeting in San Diego.
The test was shown to be 84% accurate at predicting fatty liver disease five years in advance and 76% accurate at predicting it 16 years ahead of diagnosis. When additional factors like body mass index (BMI) and daily exercise habits were added, the prediction accuracy improved even more—over 90% at five years and 82% at 16 years.
This model was tested on two different groups—participants from the UK Biobank (over 50,000 people) and a separate group in China—showing promising results across diverse populations.
Why Early Detection Matters
Fatty liver disease not only damages the liver but also increases the risk of early death—primarily from cardiovascular diseases (CVD) rather than liver failure itself. The connection between MASLD and heart disease is strong, as both share causes like high blood sugar, high triglycerides, and obesity. That is why early diagnosis is crucial—not just to protect the liver, but also to manage the risk of heart disease.
Early identification through a simple blood test could lead to earlier lifestyle changes, medical monitoring, and interventions that can prevent serious complications like cirrhosis or heart attacks.
What You Can Do
Currently, there is no approved medication for MASLD. Treatment mainly involves:
- Maintaining a healthy weight through diet and exercise
- Controlling diabetes and cholesterol levels
- Avoiding alcohol
- Following medical advice if already diagnosed
Preventive steps include eating a balanced diet rich in lean proteins, fruits, vegetables, whole grains, and healthy oils, being physically active, and avoiding unnecessary medications.
Although the findings are considered preliminary until published in a peer-reviewed journal, this research marks a major advancement. It shows that a simple blood test could soon help millions of people know their risk decades in advance, giving them a chance to change the course of the disease before it's too late.
Credits: Canva
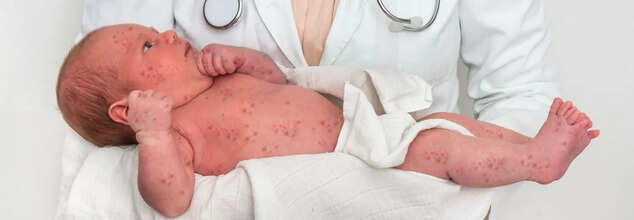
Why Is There A Growing Misinformation On Measles?
As the United States experiences the worst measles outbreak in over a decade with nearly 900 reported cases across 29 states, including deaths of two children, public understanding of the disease and its prevention strategy have been highly misunderstood due to political divide. The outbreak began in West Texas and has now spread widely. This highlights not only the persistent threat of measles but also the growing influence of vaccine misinformation, which have been fueled by political figures.
Why Is There A Resurgence of Preventable Diseases?
Measles, a highly contagious viral disease, had been largely controlled in the U.S. thanks to the widespread use of the measles, mumps, and rubella (MMR) vaccine. However, gaps in vaccination coverage have led to a resurgence. The current outbreak, centered in Texas but extending to almost 30 states, has alarmed public health officials. Two young girls who were otherwise healthy have died as a result of measles complications.
The disease is known for its rapid transmission. A single case can lead to significant spread if vaccination rates in a community fall below the 95% threshold needed for herd immunity. Children are particularly vulnerable; although the first dose of the MMR vaccine is typically administered at 12 months, increased parental concern has led some to seek earlier vaccination during the outbreak.
The Politics Of Vaccine
As per a new survey by KKF, a nonprofit health information group, only one-third of Republican-leaning parents were aware of the current measles outbreak. This was in comparison with the two-thirds of aware Democratic parents. The survey also found and 1 in 5 Republicans believes that measles vaccine is more dangerous than the diseases itself, this is double the rate of Democrats.
These gaps in perception are not new but are deepening. About 35% of Republicans surveyed believe the discredited theory that the MMR vaccine is linked to autism—a belief held by just 10% of Democrats. While belief in this theory has not increased significantly, public awareness of the claim has grown, reflecting the impact of persistent misinformation.
Adding to the confusion is Health and Human Services Secretary Robert F. Kennedy Jr., who has long been associated with anti-vaccine rhetoric. Since taking office, he has supported an investigation into environmental contributors to autism and has floated unproven theories, such as vitamin A being a preventative measure against measles. These statements, while not outright opposing vaccines, muddy public understanding and can discourage immunization.
At a time when clear public health messaging is critical, conflicting remarks from top officials are exacerbating distrust. Advocacy organizations like Immunize.org and The Immunization Partnership warn that such mixed messaging, especially when it originates from high-ranking leaders, undermines public confidence in vaccines.
In southern states like Texas, Louisiana, Arkansas, and Oklahoma, anti-vaccine activism has gained traction within state legislatures. Studies show that even lawmakers with medical backgrounds have not always supported public health measures, often influenced by political considerations and public pressure. Testimony from vaccine opponents at legislative hearings further amplifies misinformation, making it more difficult for facts to gain traction.
Meanwhile, healthcare providers are seeing the consequences firsthand. In California, where a 2014–15 outbreak began at Disneyland, pediatricians have treated severely ill children and taken stricter stances on accepting unvaccinated patients. These outbreaks serve as a stark reminder that the threat of measles is real and recurring.

Credits: Canva
IBS Awareness Month: Could Gravity Be Wrecking Your Digestive Health?
Every year in April, IBS Awareness Month puts the spotlight on one of the world's most misunderstood and elusive gastrointestinal disorders—Irritable Bowel Syndrome (IBS). Affecting about 10% of the world's population, IBS has puzzled physicians and researchers for years. Though its chronic and interruptive symptoms—abdominal pain and bloating on one end, constipation and diarrhea on the other—there remains no agreement on what actually triggers it. A new, daring theory is disrupting conventional wisdom by posing an unusual question- Is gravity the hidden culprit behind IBS?
Dr. Brennan Spiegel, a Cedars-Sinai Medical Center physician and researcher in Los Angeles, is leading the way to a new perspective on IBS—one that looks at the constant pull of gravity on our bodies. In the American Journal of Gastroenterology, Spiegel's theory is that IBS could be the body's failure to successfully deal with gravity.
It's an interesting concept. "We spend our whole life in gravity, are formed by it, but barely appreciate its constant effect on our body," Spiegel explained in an interview. "Each strand of our body is touched by gravity every day, from the top of our head to our gastrointestinal tract."
From a scientific perspective, this hypothesis borrows from evolutionary biology and neurophysiology. The human body over millennia has evolved complex systems—musculoskeletal, gastrointestinal, neurological—to deal with the downward pull of gravity. When these systems fail, Spiegel argues, they can initiate not only gastrointestinal symptoms but also a cascade of other complaints—muscle pain, fatigue, changed mood, and more.
What is the Gut-Brain Axis and the Roller Coaster Effect?
At its core is the connection between the gut and the brain—a widely documented characteristic of IBS. Individuals with IBS tend to experience a knotted stomach upon stress or a sensation of "butterflies" in stressful situations. Such gut feeling, as Spiegel speculates, may be attributed to the nervous system's adaptation to threats from gravity, such as the free-falling experience on a roller coaster.
"Our nervous system has mechanisms for perceiving and reacting to changes in gravity," he added. "When it flakes out or overcompensates, it may show up as IBS symptoms." This is related to another fascinating twist: the difference between individuals' reactions to gravitational stress. There are those who love roller coasters; others get nauseated or frantic—implying a continuum of what Spiegel refers to as G-force vigilance.
This might explain why IBS tends to overlap with disorders such as anxiety, depression, fibromyalgia, and chronic fatigue syndrome—all of which could potentially have an underlying sensitivity to gravitational stress.
Link Between IBS and Gravity
Spiegel's theory also explores deeper into anatomy. The abdominal cavity houses heavy organs that need to be "suspended" effectively. Genetic predispositions—lax connective tissues, a weak diaphragm, or spinal misalignments in some—may lead to sagging or movement of organs, including the intestines. The downward movement could affect motility, lead to cramping, and result in bacterial overgrowth—all prevalent in IBS.
In addition, the hypothesis delves into serotonin's role. This mood-regulating neurotransmitter also facilitates balance, blood circulation, and the movement of intestinal contents. "Dysregulated serotonin," Spiegel explains, "could actually be a type of gravity failure," which may connect depression, IBS, and even dizziness in a common physiological cycle.
What It Means for Treatment and Prevention
If confirmed, the gravity hypothesis has the potential to transform how we conceptualize—and treat—IBS. "The beauty of it is that it's testable," said Dr. Shelly Lu, director of the Division of Digestive and Liver Diseases at Cedars-Sinai. Unlike so many vague IBS theories, this one invites us to the possibility of targeted interventions.
This hypothesis can also assist us in better comprehending the reason why exercise, posture correction, core strengthening, and physical therapy work for most IBS sufferers. By fortifying the structural support system of the body, these strategies might decrease gravity's effect on the gut.
Symptoms, Triggers, and the IBS Daily Struggle
IBS is an individualized disease. Symptoms range widely and may include:
- Abdominal bloating, pain, and cramping related to bowel movements
- Alterations in stool appearance or frequency
- A feeling of not fully emptying the bowels, gas, and mucus in stool
Its triggers are also multifactorial. Stress and some foods—dairy, citrus, beans, wheat, and carbonated beverages—may exacerbate symptoms. Although food intolerances are not the cause in every case, many individuals find significant symptom relief through diet, such as a low FODMAP diet.
IBS isn't only an intestinal affliction—it can also blow a person's life off track. Chronic patients usually suffer from complications such as hemorrhoids due to constant diarrhea or constipation, and generally decreased quality of life. Research indicates that individuals with moderate to severe IBS experience three times more work absences compared to others. The psychological price is high too—aún and depression often accompany one another in IBS, one worsening the other.
Could this be the long-lost piece to a hundred-year-old puzzle? Maybe. Although further research is necessary to establish the gravity connection, the theory is already creating new avenues for comprehension and healing.
If you have ongoing digestive problems, see a healthcare provider. IBS is a treatable condition, and treatment options can involve medication, counseling, physical therapy, or dietary changes depending on your individual symptoms.
© 2024 Bennett, Coleman & Company Limited

